Answers
The weight of an alcohol with a density of 0.8g/ml and volume of 50ml is 40g.
HOW TO CALCULATE WEIGHT:
The weight of a substance can be calculated by multiplying its density by volume. That is;Mass = density × volumeAccording to this question, alcohol has a density of 0.8g/ml and a volume of 50ml. The weight is as follows:Weight = 0.8g/ml × 50mlWeight = 40gTherefore, the weight of an alcohol with a density of 0.8g/ml and volume of 50ml is 40g.Learn more at: https://brainly.com/question/23245710?referrer=searchResults
Related Questions
Becca is a forensic technician analyzing the fragments of a window. She sees that there is a hole in the window, and that the outside hole is smaller than the inside hole. What might she deduce from this information?
Answers
The observation of a smaller outside hole than inside leads Becca to infer that an impact from the outside caused the hole, with a larger object striking and passing through the window from the inside.
From the observation that the hole in the window is smaller on the outside than on the inside, Becca, as a forensic technician, might deduce the following:
The hole was caused by an impact from the outside: The smaller outside hole suggests that the force that created the hole originated from the outside and exerted more pressure on the window surface facing inward.
The object causing the hole was larger on the inside: The discrepancy in hole sizes implies that the object that struck the window had a larger size or diameter on the inside, and as it penetrated the glass, it compressed or fragmented the glass, resulting in a larger hole on the inside.
The object may have passed through the window: The difference in hole sizes indicates that the object may have penetrated the window, potentially passing through to the inside. This could suggest a break-in or an incident involving the window being struck from the outside.
Overall, the observation of a smaller outside hole than inside leads Becca to infer that an impact from the outside caused the hole, with a larger object striking and passing through the window from the inside.
For more question on observation
https://brainly.com/question/29521469
#SPJ8
Why does ear escape from a tire when a tire valve is opened
Answers
Air escape from a tire when tire valve is opened because the pressure from the weight of the car is forcing the already pressurized air out .
Why does air escape from tire when tire valve is opened?When air is filled in tires, it get into more compact area than the outer atmosphere, therefore particles in tire are close to each other and exert pressure continuously on tire walls to get out of it. Thus, material of the tire need to be pressure resistant as much possible.
After opening the tire valve, air starts leaving with huge sound as strain outside the tire is weaker than that inside the tire. Molecules strike with the same force on larger area and pressure starts diminishing.
To know more about tire valve, refer
https://brainly.com/question/3720473
#SPJ1
Note: The question given on the portal is incomplete. Here is the complete question.
Question: Air leaves a tire when the tire valve is opened because
A. the pressure outside the tire is lower than the pressure inside the tire.
B. the pressure outside the tire is greater than the pressure inside the tire.
C. the temperature is higher outside the tire than inside the tire.
D. there are more gas particles outside the tire than inside the tire.
What is the molarity of a solution that contains 0.180 moles KOH in 0.350 L of solution?
Answers
Answer:
molarity of solution = 0.514
Explanation:
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Hello please help, thank you so much!

Answers
Answer:
7.) 126.7 cm³
8.) 789 g
Explanation:
7.) To find the volume (cm³) of alcohol, you need to multiply the given mass (g) by the density. The denisty ratio represents grams of the alcohol per every 1 cm³. It is important to arrange the ratio in a way that allows for the cancellation of units (grams should be in the denominator). The final answer should have 4 sig figs to match the given value with the least amount of sig figs (100.0 grams).
100.0 grams 1 cm³
--------------------- x --------------------------- = 126.7 cm³
0.78945 grams
8.) This time, you are given the volume instead of the mass. The same process can be used in this problem, but you first need to convert liters (L) to cm³ to be able to use the denisty ratio. Once you find the volume in cm³, you can multiply it by the density to find the mass. Again, be sure to arrange the conversions in a way that allows for the cancellation of units. The final answer should have 3 sig figs to match the given value with the least amount of sig figs (1.00 L).
1 L = 1,000 cm³
1.00 L 1,000 cm³ 0.78945 g
------------- x ------------------- x -------------------- = 789 g
1 L 1 cm³
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
calculate the volume of hydrogen in the reaction of 73 grams of zinc and 73 grams of hydrochloric acid (under normal conditions) please help
Answers
The volume of hydrogen gas produced in the reaction of 73 grams of zinc and 73 grams of hydrochloric acid (under normal conditions) is approximately 22.4 liters.
To calculate the volume of hydrogen gas produced in the reaction of zinc and hydrochloric acid, we need to use the principles of stoichiometry and the ideal gas law.
First, let's write the balanced chemical equation for the reaction between zinc (Zn) and hydrochloric acid (HCl):
Zn + 2HCl →\(ZnCl_2\)+ H2
From the equation, we can see that one mole of zinc reacts with two moles of hydrochloric acid to produce one mole of hydrogen gas. To determine the number of moles of zinc and hydrochloric acid, we need to convert the given masses into moles.
The molar mass of zinc (Zn) is approximately 65.38 g/mol, so 73 grams of zinc is equal to:
73 g Zn * (1 mol Zn / 65.38 g Zn) ≈ 1.116 mol Zn
Similarly, the molar mass of hydrochloric acid (HCl) is approximately 36.46 g/mol, so 73 grams of HCl is equal to:
73 g HCl * (1 mol HCl / 36.46 g HCl) ≈ 2.002 mol HCl
According to the balanced equation, the reaction produces one mole of hydrogen gas for every two moles of hydrochloric acid. Therefore, since we have 2.002 moles of HCl, we expect to produce half that amount, or approximately 1.001 moles of hydrogen gas.
To calculate the volume of hydrogen gas, we can use the ideal gas law, which states:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature. In this case, we assume the reaction is conducted under normal conditions, which means a pressure of 1 atmosphere and a temperature of 273.15 Kelvin.
Rearranging the equation to solve for V, we have:
V = nRT / P
Substituting the values, we get:
V = (1.001 mol) * (0.0821 L·atm/(mol·K)) * (273.15 K) / (1 atm) ≈ 22.4 L
Therefore, the volume of hydrogen gas produced in the reaction is approximately 22.4 liters.
For more such information on: volume
https://brainly.com/question/29796637
#SPJ8
Formation of crystals of sugar from a sugary syrup is a …….
chemical change
Answers
It's a chemical change.
Explanation:-
Formation of crystals of sugar from a sugary syrup is a chemical change. Because, we cannot get sugary syrup back from the sugar crystals. Yet, it is chemical change.
how many unpaired electron are there is Co2+ ion
Answers
Answer:
There is 3 unpaired electron in Co2+ Ion⊂⊃: explanation is the picture above
#CarryOnLearning
#BetterWithBrainly

What amount of heat, in kJ, is required to vaporize 181.20 g of ethanol (C₂H₅OH)? (∆Hvap = 43.3 kJ/mol)
Answers
The amount of heat required to vaporize 181.20 g of ethanol would be 170.1 kJ.
Heat of vaporizationUsing the formula:
Q = n ∆Hvap
where:
Q is the amount of heat required to vaporizen is the number of moles of the substance∆Hvap is the molar heat of vaporization.Moles of 181.20 g of ethanol = 181.20 g / 46.07 g/mol = 3.933 mol
Substituting the values:
Q = 3.933 mol x 43.3 kJ/mol = 170.1 kJ
In other words, the amount of heat required to vaporize 181.20 g of ethanol is 170.1 kJ.
More on heat of vaporization can be found here: https://brainly.com/question/30603212
#SPJ1
Name the four states of matter, and a characteristic to describe each.
Answers
Answer:
solid particles are tightly held.
liquid particles have perfectly inelastic collisions
gas move in constant random motion
vapour
electronic configuration of organic compounds
Answers
The electronic configuration of organic compounds depends on the orbitals of their atoms and molecules.
What is electronic configuration?The expression 'electronic configuration' makes reference to the spacial arrangement of electrons in distinct energy orbitals of an atom/molecule.
The orbitals are designed with numbers and letters, whereas the amount of electrons in each orbital is expressed as superscripts (e.g., 1s² 2s² 2p² in the C atom that form glucose).
In conclusion, electronic configuration of organic compounds depends on the orbitals of their atoms and molecules.
Learn more about electronic configurations here:
https://brainly.com/question/26084288
#SPJ1
Where do stars form?
Answers
Stars form in large, dense regions of gas and dust known as molecular clouds. These clouds are located primarily in the spiral arms of galaxies, where they are exposed to intense radiation from nearby stars. As the gas and dust in these clouds are subjected to this radiation, they begin to collapse under their own gravity. As the collapse continues, the cloud becomes denser and denser, and eventually a protostar forms at its center. Over time, this protostar continues to contract and heat up, eventually reaching the point where nuclear fusion can begin in its core. At this point, the protostar becomes a fully-fledged star, and the process of star formation is complete.
TL;DR: Within the clouds of dust and scattered throughout most galaxies.
A reaction occurs in a calorimeter, resulting in the starting temperature of 38.8 ℃ and final temperature 21.0 ℃. What can you say about the reaction and the enthalpy change (ΔH) during the reaction?
Answers
Answer:
hola comoe stas
Explanation:
gracias x los puntos
In the following take CV = 20.8 and CP = 29.1 J⋅mol−1⋅°C−1 for nitrogen gas: (a) Three moles of nitrogen at 30°C, contained in a rigid vessel, is heated to 250°C. How much heat is required if the vessel has a negligible heat capacity? If the vessel weighs 100 kg and has a heat capacity of 0.5 kJ⋅kg−1⋅°C−1, how much heat is required? (b) Four moles of nitrogen at 200°C is contained in a piston/cylinder arrangement. How much heat must be extracted from this system, which is kept at constant pressure, to cool it to 40°C if the heat capacity of the piston and cylinder is neglected?
Answers
Answer:
\(224 \times 13313\frac{.131?}{?244} \)
I need help with this

Answers
As a result, the ideal gas law is applied, and the pressure of the gas in the container is 1.44 atm.
How does Charles Law compute pressure?The Kelvin temperature and hence the volume are going to be in direct proportion when the pressure on a sample of a dry gas is held constant, according to the definition of the Charles Law Formula. PV = k is the law's equation, and k might be a constant.
This issue can be resolved by applying the ideal gas law:
PV = nR
T = -52 °C + 273.15 = 221.15 K
n = 0.642 mol
V = 8.6 L
T = 221.15 K
\(R = 0.0821 L·atm/mol·K (gas constant for ideal gases)\)
PV = nRT
P = nRT/V
P = (0.642 mol)(0.0821 L·atm/mol·K)(221.15 K)/(8.6 L)
P = 1.44 atm
To know more about ideal gas visit:-
https://brainly.com/question/28257995
#SPJ1
Which of the following is not a compound?
Responses A.H2O b.CO2 C. N2 D.CH4
Answers
Compounds are formed by the combination of atoms of different elements. Molecules are formed by atoms of same elements. Thus, N₂ is a molecule not a compound.
What are compounds?Compounds are formed by the combination of atoms of different elements. There are different kinds of compounds such as ionic compounds, covalent compounds.
For example, water, H₂O is a compound formed from two hydrogen atoms and oxygen atom. Similarly carbon dioxide or CO₂ is a covalent compound formed from two oxygens and one carbon atom. Methane or CH4 also is a compound.
N₂ is a molecule and not a compound. It is formed by the combination of two equivalent nitrogen atoms. Therefore, option C is correct.
To find more on compounds, refer here:
https://brainly.com/question/13516179
#SPJ1
What indicates that two objects are in thermal equilibrium?
A The Objects Are the same size
B The objects Have the same temperature
C The objects are touching each other.
D The objects' temperatures are changing.
Answers
Answer:
B
Explanation:
equilibrium happens when two things are in complete balance and thermal is in reference to heat so two things are in thermal equilibrium once they have the same temperature.
The option which indicates that two objects are in thermal equilibrium is the objects that have the same temperature. Thus, the correct option for this question is B.
What is Thermal equilibrium?Thermal equilibrium may be defined as a state of a system in which all the parts of the system occupy the same temperature. Two or more objects are in thermal equilibrium if there is no net flow of thermal energy or heat between them when they are considerably connected by a path permeable to heat.
The mechanism of thermal equilibrium occurs when a cup of hot coffee is put on the table and after a short period of time, the temperature of the coffee is the same as the surrounding temperature. So, this represents an example of thermal equilibrium.
Therefore, the correct option for this question is B.
To learn more about Thermal equilibrium, refer to the link:
https://brainly.com/question/9459470
#SPJ2
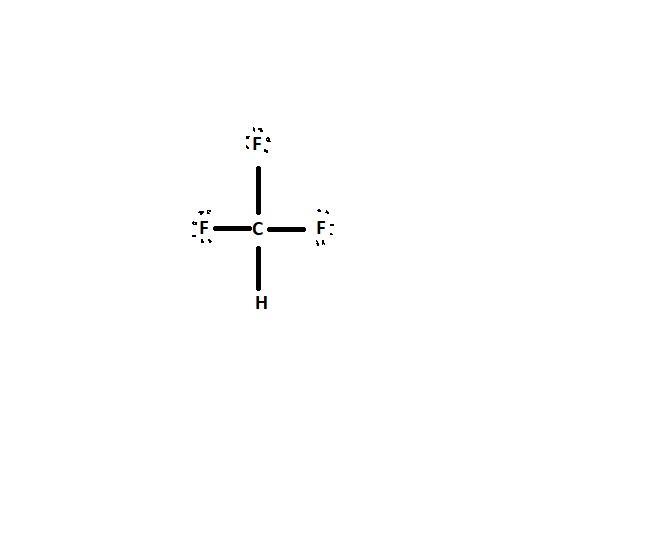
how to do the lewis diagram of CHF3
Answers
A lewis structure is a simplified form of representing valence electrons of a compound.
For the compound CHF3, we will follow the steps below to write its lewis diagram
Step 1: Add4 valence electrons around the carbon atom as shown:
Step 2: Add dots to the Fluorine atom and hydrogen atom
Step 3: Combine the two steps above to have:
This gives the required lewis diagram for CHF3


Lewis dot structure refers to the presence of valence electrons in molecules, either in the form of lone pairs or in the form of bonds.
For example, CHF₃ has a Lewis dot structure that consists of a central carbon atom (1), 3 fluorine atoms (3), and 1 hydrogen atom (1) as terminal atoms.
In a Lewis dot structure, the least electroconductive atom (other than Hydrogen) of the molecule is represented as the central atom. For example, in the periodic table, carbon is less electroconductive than fluorine, because electroconductivity increases from left to right.
The image of the Lewis structure of CHF₃ is attached below.
To learn more about the valence electrons, refer to the link:
https://brainly.com/question/31264554
#SPJ6
jalyn ran at speed of 3 m/s then came to a sudden stop during a time period of 3 s. what was his acceleration
Answers
Answer:
I believe the answer is 1m/s²
Explanation:
acceleration= change in speed ÷ time (I hope this is correct)
What would be the volume in mL of 5.097 g of water delivered by a 5 mL pipet? You observe the temperature and find the density to be 0.9960 g/mL. Do not use units in answer and record to 3 significant figures.
Answers
The volume of the water is 5.11 mL.
What would be the volume?Density is a measure of mass per unit of volume of a substance. It is a physical property of matter and is expressed in units of grams per cubic centimeter (g/cm^3).
The density of a substance determines how much of it will occupy a given space, and is a crucial factor in many physical and chemical phenomena, such as buoyancy, phase changes, and thermal conductivity.
We have that;
Volume of the water = ?
Density of the water = 0.9960 g/mL
Mass of the water = 5.097 g
Volume of the water = Mass/Density
= 5.097 g / 0.9960 g/mL
= 5.11 mL
Learn more about density:https://brainly.com/question/29775886
#SPJ1
If a sample of a gas at 32°C has a pressure of 1.9 to atm what will its pressure be at 78°C
Answers
The pressure of the sample gas at 78°C is 2.17 atm
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. Volume is a degree of occupied three-dimensional space. it's far more frequently quantified numerically the usage of SI-derived gadgets or by way of diverse imperial gadgets. The definition of length is interrelated with the extent.
An ideal gas is described as one for which both the extent of molecules and forces between the molecules are so small that they have got no effect on the behavior of the gas.
The real gas that acts almost like a really perfect gasoline is helium. that is due to the fact helium, in contrast to maximum gases, exists as an unmarried atom, which makes the van der Waals dispersion forces as low as viable
Using the ideal gas equation:-
Given;
P₁ = 1.9 atm
T₁ = 32°C = 305 K
P₂ = ?
T₂ = 78°C = 351 K
P₁/T₁ =P₂/T₂
P₂ = P₁T₂ /T₁
= 1.9 × 351 / 305
= 2.17 atm
Learn more about ideal gas here:-https://brainly.com/question/20348074
#SPJ9
A chemist weighs out 310 grams of selenium
tetrafluoride, SeF4, a liquid used as a fluorinating
reagent. How many moles of the compound have
been weighed?
0.50 mole
O 1 mole
2 moles
48050 moles
Write out all the steps

Answers
A chemist weighs out 310 grams of selenium tetrafluoride, and 2 moles of selenium tetrafluoride (SeF₄) have been weighed. Therefore, option C is correct.
Number of moles = Mass of the compound / Molar mass of the compound
The molar mass of selenium = 78.96 g/mol
The molar mass of fluorine (F) = 18.998 g/mol.
Since SeF₄ contains one selenium atom and four fluorine atoms, the molar mass of SeF₄ is:
Molar mass of SeF₄ = (1 × Molar mass of Se) + (4 × Molar mass of F)
Molar mass of SeF₄ = (1 × 78.96 g/mol) + (4 × 18.998 g/mol)
Molar mass of SeF₄ ≈ 156.96 g/mol
Now, calculate the number of moles:
Number of moles = 310 g / 156.96 g/mol
Number of moles ≈ 2 moles
To learn more about the moles, follow the link:
https://brainly.com/question/30885025
#SPJ1
Aanlyze the potential energy diagram of the
reaction shown.
Which statements about the reaction are true?
Select all that apply.
The reaction is exothermic
The reaction is endothermic
The equation shows that 891 kJ of energy are
released as a product.
The equation shows that 891 kJ of energy are
absorbed as a reactant.
The graph shows that the reactants have greater potential energy than the products.
The answer is a,c,e

Answers
Answer:
A: The reaction is exothermic
C: The equation shows that 891 kJ of energy are released as a product.
E: The graph shows that the reactants have greater potential energy than the products.
Explanation:
The statement about the reaction is true:
A: The reaction is exothermic
C: The equation shows that 891 kJ of energy is released as a product.
E: The graph shows that the reactants have greater potential energy than the products.
What is potential energy?Potential energy is the energy that is saved in the body. It is used in the work and when convert into kinetic energy.
A moving item fundamentally possesses kinetic energy. Kinetic energy would therefore be created as a result of an object moving or an elastic band being released since the released object exhibits motion.
Therefore, the correct option is A: The reaction is exothermic, C: The equation shows that 891 kJ of energy is released as a product, and E: The graph shows that the reactants have greater potential energy than the products.
To learn more about potential energy, refer to the link:
https://brainly.com/question/15764612
#SPJ2
an iron ( Fe ) bar that loses 2.42 kJ when its temperature decreases from 252 ∘C to 79 ∘C
Answers
The calculated mass is 35.6.
The movement of minuscule atoms, molecules, or ions in solids, liquids, and gases produces heat energy. From one thing to another, heat energy can be exchanged. Heat is the flow or transfer that occurs as a result of the temperature differential between two objects.
Q = m*c*ΔT,
ΔT = Initial T - Final T
For iron, the specific heat capacity is 0.450 J/(g°C) and ΔT Q is the heat energy in calories (2.92 KJ = 2920 Joules).
The mass of water is m.
The values 2920 J= m* 0.450 J/g°C * are entered (254 °C-72 °C)
The solution is mass=2920 J/ 0.450*180°C
mass = 35.6.
Learn more about mass here-
https://brainly.com/question/19694949
#SPJ9
How did modern scientists experiment with Van Goghs paints to determine their chemical reactions?
Answers
Answer:
the would test random expirements
What does voltage describe?
Answers
The Voltage is the pressure from the electrical circuit of the power source that passes the current.
The Voltage is defined as the pressure from the electrical circuit of the power source that will passes the charged electrons that is the current through the conducting loop, it will enable them to do work because of the illuminating the light. The in simple terms is : voltage = pressure, and it is denoted as the volts and the symbol is the V.
The voltage is described as the force that causes the flow of the charged particles. The Voltage is also called as the electromotive force.
To learn more about voltage here
https://brainly.com/question/13177389
#SPJ1
Pls help i have 0 clue what this even means
Show all work including units and the equation you used to solve. Carbon dioxide gas has a molar mass of 44 g/mol. At 300K and 1.5atm, a sample of carbon dioxide has a volume of 4.5 L. Find the number of moles of the carbon dioxide.
EXTRA POINTS: Find the mass of the carbon dioxide.
Answers
Answer: 0.27 moles of CO2 and 11.88 grams of CO2
Explanation: Use the Ideal Gas Law, PV = nRT, substitute the values given and solve.
I can't seem to upload procedure but:
P = 1.5atm
V = 4.5L
n = moles
R = 0.0821gr/mol (when using atm, kPa is 8.31)
T = 300K
Isolate what you don't have, in this case n. Change PV = nRT to PV/RT = n. Substitute the values to get moles. Once you have this, multiply the value by the molar mass of CO2 (44gr/mol) to get the mass of CO2 in grams.
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
There are four molecules of nitrogen and nine molecules of hydrogen present in the diagram.
When the reaction is complete, how many molecules of NH3 are produced?
What is the limiting reactant?
How many molecules of each reactant are remain after the reaction is complete?
Answers
After the reaction is complete, no nitrogen and no hydrogen molecules remain, and 8.00 x 1014 molecules of NH3 are produced.
In the equation, nitrogen and hydrogen react at a high temperature, in the presence of a catalyst, to produce ammonia, according to the balanced chemical equation:N2(g)+3H2(g)⟶2NH3(g)The coefficients of each molecule suggest that one molecule of nitrogen reacts with three molecules of hydrogen to create two molecules of ammonia.
So, to determine how many molecules of ammonia are produced when four nitrogen and nine hydrogen molecules are present, we must first determine which of the two reactants is the limiting reactant.
To find the limiting reactant, the number of moles of each reactant present in the equation must be determined.
Calculations:
Nitrogen (N2) molecules = 4Hence, the number of moles of N2 = 4/6.02 x 1023 mol-1 = 6.64 x 10-24 mol
Hydrogen (H2) molecules = 9Hence, the number of moles of H2 = 9/6.02 x 1023 mol-1 = 1.50 x 10-23 mol
Now we have to calculate the number of moles of NH3 produced when the number of moles of nitrogen and hydrogen are known, i.e., mole ratio of N2 and H2 is 1:3.
The mole ratio of N2 to NH3 is 1:2; thus, for every 1 mole of N2 consumed, 2 moles of NH3 are produced.
The mole ratio of H2 to NH3 is 3:2; thus, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
From these mole ratios, it can be observed that the limiting reactant is nitrogen.
Calculation for NH3 production:
Nitrogen (N2) moles = 6.64 x 10-24 moles
The mole ratio of N2 to NH3 is 1:2; therefore, moles of NH3 produced is 2 × 6.64 × 10−24 = 1.33 × 10−23 moles.
Now, to determine how many molecules of NH3 are produced, we need to convert moles to molecules.
1 mole = 6.02 x 1023 molecules
Thus, 1.33 x 10-23 moles of NH3 = 8.00 x 1014 molecules of NH3 produced.
To find the amount of each reactant remaining after the reaction is complete, we must first determine how many moles of nitrogen are consumed, then how many moles of hydrogen are consumed, and then subtract these from the initial number of moles of each reactant.
The moles of nitrogen consumed = 4 moles × 1 mole/1 mole N2 × 2 mole NH3/1 mole N2 = 8 moles NH3
The moles of hydrogen consumed = 9 moles × 2 mole NH3/3 mole H2 × 2 mole NH3/1 mole N2 = 4 moles NH3
Thus, the moles of nitrogen remaining = 6.64 × 10−24 mol – 8 × 2/3 × 6.02 × 10^23 mol-1 = 5.06 × 10−24 mol
The moles of hydrogen remaining = 1.50 × 10−23 mol – 4 × 2/3 × 6.02 × 10^23 mol-1 = 8.77 × 10−24 mol
Finally, the number of molecules of each reactant remaining can be calculated as follows:
Number of N2 molecules remaining = 5.06 × 10−24 mol × 6.02 × 10^23 molecules/mol = 3.05 × 10−1 molecules ≈ 0 molecules
Number of H2 molecules remaining = 8.77 × 10−24 mol × 6.02 × 10^23 molecules/mol = 5.28 × 10−1 molecules ≈ 0 molecules.
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
What effects does human population have on forest trees
Answers
Explanation:
Depreciation on fixed assets is recorded in the accounts of:
general fund D. capital projects fund
enterprise fund E. debt service fund
special Revenue fund