the covalent bond formed between two amino acids is called a(n) a. glycosidic bond b. peptide bond c. phosphodiester bond d. ester bond e. hydrolytic bond
Answers
The covalent bond formed between two amino acids is called a peptide bond.
Peptide bonds are formed by the condensation reaction between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another, resulting in the release of a molecule of water (H2O). This reaction forms a covalent bond, known as a peptide bond, which links the two amino acids together in a linear sequence.
Peptide bonds are critical in the formation of polypeptides and proteins, as they determine the sequence and structure of these biomolecules. The peptide bond is a single bond, and its formation and stability are due to the partial double bond character that it possesses. This partial double bond character is due to the delocalization of electrons in the bond and the contribution of resonance from the atoms on either side of the bond.
In summary, a peptide bond is a type of covalent bond that forms between two amino acids and is critical in the formation and stability of polypeptides and proteins.
Learn more about peptide bond:
brainly.com/question/28295128
#SPJ
Related Questions
832 J of energy is used to raise the temperature of an unknown metal from 65oC to 71oC. If the specific heat of the metal is 0. 466 J/g*C, what is the mass of the metal sample? g (five sig figs)
Answers
The formula for calculating the amount of energy required to raise the temperature of a substance is:
q = m * c * ΔT
where q is the amount of energy, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
We can rearrange this formula to solve for the mass of the metal:
m = q / (c * ΔT)
Substituting the given values, we get:
m = 832 J / (0.466 J/g*C * (71oC - 65oC))
m = 832 J / (0.466 J/g*C * 6oC)
m = 832 J / 2.796 J/g
m = 297.1387678 g
Rounding to five significant figures, the mass of the metal sample is 297.14 g.
To know more about substance refer here
https://brainly.com/question/13320535#
#SPJ11
How many moles of NaOH are contained in 700. Ml of 3. 00 M NaOH solution?
Answers
The number of moles of NaOH contained in 700ml of 3.00 M concentration of NaOH are 2.1 moles.
To calculate the number of moles of NaOH in a 700 mL of 3.00 M NaOH solution, we can use the following formula:
moles = concentration (M) x volume (L)
First, we need to convert the volume from milliliters (mL) to liters (L):
700 mL = 0.7 L
Now we can plug in the values we know:
moles = 3.00 M x 0.7 L
moles = 2.1 moles
Therefore, there are 2.1 moles of NaOH in 700 mL of 3.00 M NaOH solution.
According to Avogadro's number, one mole (abbreviated as mol) equals 6.022 x 10²³ molecular entities. Each element has a unique molar mass based on the weight of 6.022 x 10²³ of its atoms.
To know more about number of moles, refer:
https://brainly.com/question/23991631
#SPJ4
describe how the osmotic tolerance of an organism such as Staphylococcus differs from the osmotic tolerance of E.coli. What cellular structure is this difference based on?
Answers
The osmotic tolerance of an organism such as Staphylococcus differs from the osmotic tolerance of E.coli as well as the cellular structure difference that is based on is given below:
Enterococci and Escherichia coli are less salt tolerant than staphylococci. Their cell walls are more stiff, and their internal turgor pressure is higher.
How do e coli cells respond to osmotic changes?In response to hyperosmotic shock, E. coli imports potassium and, if available, suitable solutes such proline and glycine betaine (27).
Most strains of Staphylococcus aureus grow well in a high-NaCl concentration media containing as much as 15% NaCl because it is a salt-tolerant eubacterium (1).
Therefore, Osmotolerant microorganisms are those that can accomplish this and so endure hypertonic conditions. Osmotolerant microorganisms, including Staphylococcus aureus, may thrive in a wide range of osmotic pressures and conditions. In fact, media containing sodium chloride (NaCl) concentrations up to 3M can be used to cultivate this bacteria.
Learn more about Staphylococcus from
https://brainly.com/question/8050491
#SPJ1
Using the periodic table, calculate the number of protons, neutrons, and electrons for Calcium, Argon, and Bromine.(Ca,Ar,Br)
Answers
Answer:
Calcium has 20 protons 26 neutrons and 20 electrons.
argon has 18 protons
22 neutrons and 18 electrons
Bromine has 35 protons 44 neutrons and 35 electonrs
Explanation:
good luck with your homework hope I helped
Which energy source is considered nonrenewable?
1) moving water
2) fossil fuel
3) wind
4) biomass
Answers
Answer:
Fossil fuel
Explanation:
Answer:
2
Explanation:
Nonrenewable energy resources include coal, natural gas, oil, and nuclear energy. Once these resources are used up, they cannot be replaced, which is a major problem for humanity as we are currently dependent on them to supply most of our energy needs.
Select two correct answers.
[]class
[]organism
[] fungi
[]kingdom
![Select two correct answers.[]class[]organism[] fungi[]kingdom](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/Gl3UYgFxvoHTAfrYI0rb39A7VwvcvFRb.png)
Answers
His major groupings in the hierarchy of groups were, the kingdom, phylum, class, order, family, genus, and species; seven levels of groups within groups.
Complete the formula below for the application of KVL around i1 (please ensure each term in your summation represents the voltage in V. Use the symbols I1 and I2 for the mesh currents in mA ) KVL around loop i1 : =0 V ii. Write the equation for the application of KVL around i2 KVL around loop =0 V iii. Determine the current i1 : mA iv. Determine the current i2 : mA v. Determine Vo :
Answers
the formula below for the application of KVL around i1 is KVL around loop i1: -10V + 5Ω * (I1 - I2) + 10Ω * I1 = 0.
According to Kirchhoff's Voltage Law (KVL), the sum of the voltages around a closed loop in a circuit is equal to zero. In this case, we are applying KVL around loop i1. The equation represents the sum of voltage drops in the loop, where -10V is a voltage source, 5Ω * (I1 - I2) represents the voltage drop across the 5Ω resistor due to the difference in mesh currents I1 and I2, and 10Ω * I1 represents the voltage drop across the 10Ω resistor due to the current I1. The equation is set equal to zero to satisfy KVL.
ii. KVL around loop i2: 10Ω * I2 - 5Ω * (I1 - I2) + 2V = 0
Similar to i, this equation represents the application of KVL around loop i2. The 10Ω * I2 term represents the voltage drop across the 10Ω resistor due to the current I2, the -5Ω * (I1 - I2) term represents the voltage drop across the 5Ω resistor due to the difference in mesh currents I1 and I2, and the 2V term represents a voltage source. The equation is set equal to zero to satisfy KVL.
iii. Determine the current i1: Substitute the obtained values into the equation for i1 and solve for I1 in mA.
iv. Determine the current i2: Substitute the obtained values into the equation for i2 and solve for I2 in mA.
v. Determine Vo: Substitute the obtained values for i1 and i2 into the appropriate equation representing the voltage at Vo, and calculate the value in V.
learn more about Kirchhoff's Voltage, here
https://brainly.com/question/30400751
#SPJ4
When iron combines with oxygen gas and forms rust, the total mass of the products...
is the same as the mass of the reactants.
is less than the mass of the reactants.
depends on the reaction conditions.
is greater than the mass of the reactants.
Answers
describe the smell of Na+ and H+
Answers
Answer:
Sodium ion ( Na+) is known to have no smell at all but however appears salty which is the reason why the compound Sodium Chloride has the same type of taste.
Hydrogen ions ( H+) are known to have no taste which implies it being tasteless. It is also important to note that it has no smell too when perceived (odorless).
write the balanced nuclear equation for the formation of 241 am 95 through β− decay.
Answers
The balanced nuclear equation for the formation of 241Am-95 through β− decay can be represented as follows: 94Pu-241 → 95Am-241 + -1e0
In this β− decay process, a neutron in the nucleus of 241Pu-94 is transformed into a proton, while simultaneously emitting an electron (β− particle) and an antineutrino. This leads to the formation of 241Am-95. The atomic number (Z) of the parent nucleus increases by one, resulting in the formation of a new element, americium (Am), with atomic number 95. The mass number (A) remains the same, indicating that the total number of nucleons (protons and neutrons) in the nucleus is conserved. The -1e0 in the equation represents the β− particle emitted during the process.
Learn more about β− decay process here: brainly.com/question/24141922
#SPJ11
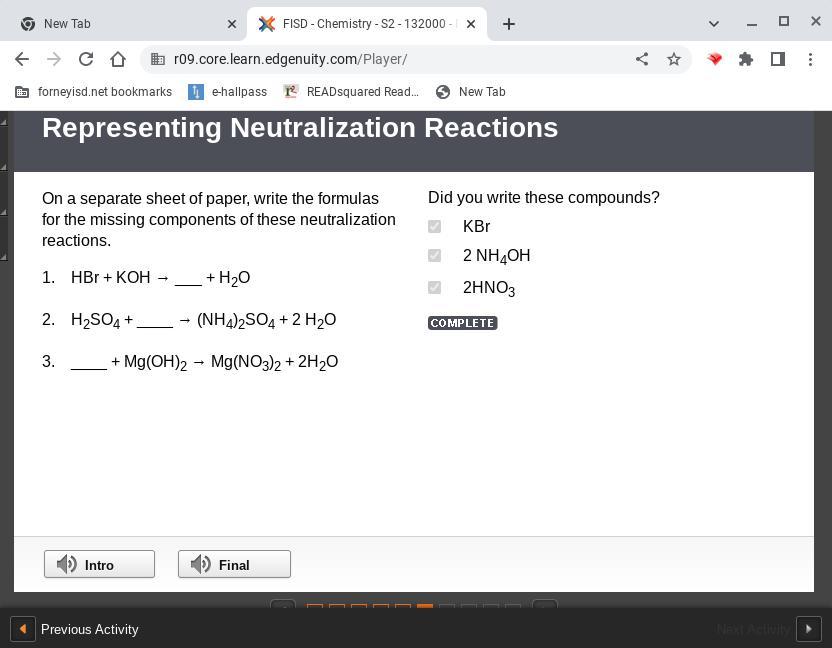
On a separate sheet of paper, write the formulas for the missing components of these neutralization reactions. 1. hbr koh → ___ h2o 2. h2so4 ____ → (nh4)2so4 2 h2o 3. ____ mg(oh)2 → mg(no3)2 2h2o
Answers
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
What is a neutralization reaction?A neutralization reaction is a reaction that occurs between an acid and a base to yield salt and water only.
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
Learn more about neutralization:https://brainly.com/question/15395418
#SPJ4
Answer:
KBr
2 NH4OH
2 HNO3
Explanation:
Express each of the following in standard form.
3.6 x 101
6.452 x 102
8.77 x 10-1
6.4 x 10-3
Answers
Answer:3.6 x 101 or 8.77 x 10-1
What does it mean when an element is reduced? It empties a valance shell, reducing its atomic radius. It gains electrons, reducing its overall charge. It increases electronegativity, reducing its ability to bond. It loses electrons, reducing its electron number.
Answers
Answer:
It gains electrons, reducing its overall charge
Explanation:
When an element is reduced, It means It gains electrons, reducing its overall charge. Hence the correct option is (B)
What is Reduction ?
Redox is a type of chemical reaction in which the oxidation states of substrate change.
Oxidation is the loss of electrons or an increase in the oxidation state (Charge) of a chemical or atoms within it.
Reduction is the gain of electrons or a decrease in the oxidation state (Charge) of a chemical or atoms within it.
Therefore, When an element is reduced, It means It gains electrons, reducing its overall charge. Hence the correct option is (B)
Learn more about Redox reaction here ;
https://brainly.com/question/13293425
#SPJ2
based on results presented in the passage, researchers hoping to alter the appearance of sgbp while maintaining its function as a cp providing a colored appearance would most logically choose to mutate which sgbp residue?
Answers
The nucleotide sequence of an organism's genome, that of a virus, extrachromosomal DNA, or other genetic components can change permanently in a process known as mutation.
Any alteration to a cell's DNA sequence. Mistakes in cell division can result in mutations, as can exposure to environmental DNA-damaging substances.
Gene mutations can be divided into two categories: small-scale mutations and large-scale mutations.
Appearance Alteration is the capacity to modify another person's skin, hair, and vocal chords (also known as adaptive appearance manifestation).
The genes that encode our pigment's sensitivity to color can multiply themselves throughout time. The additional copies are susceptible to mutations that change the range of wavelengths they can absorb.
To know about function https://brainly.com/question/11624077
#SPJ4
The chemical equation below represents one of the most important reactions for life on Earth. It describes how plants (and some microorganisms) combine water, sunlight, and carbon dioxide to produce the main source of food for most living organisms, glucose. A byproduct of this reaction is the oxygen we breathe. Is this reaction balanced? You must explain your reasoning for full credit!
PLS HELP, 30 Points!
I also need to know what I need to add to the equation to balance it!

Answers
The reaction is not balanced because the number of atoms of the elements on the reactant side isn't the same or equal to that on the product side.
What is Photosynthesis?This is referred to as the process in which green plants manufacture their food in the presence of sunlight and other compounds and is why they are regarded as primary producers in the ecosystem.
The balanced equation for photosynthesis is:
6CO₂ + 6H₂O + light --> C₆H₁₂O₆ + 6O₂
In this, we can see that the number of atoms of the elements of Carbon, hydrogen and oxygen are 6, 12 and 18 which is the same on the reactant and product side which is why the equation given in the question isn't balanced.
Read more about Photosynthesis here https://brainly.com/question/19160081
#SPJ1
A gas sample in a rigid container at 455 k is cooled to 273 k where it has a pressure of 1 atm. What was the original pressure of the gas in mmHg
Answers
Answer:
1270 mmHg
Explanation:
Answer : 1270 mmhg
Explanation:

what is entropy in chemistry
Answers
Randomness. It is simply the measure of disorder.
A sample of hydrogen at 47°C exerts a pressure of 106 kPa. The gas is heated to 77°C
at constant volume. What will its new pressure be? What law will you use?
Answers
Answer:
We can use Gay-Lussac's Law to solve this problem, which states that the pressure of a gas is directly proportional to its temperature, provided the volume and the number of moles of the gas are constant.
Using this law, we can write:
P1/T1 = P2/T2
where P1 is the initial pressure, T1 is the initial temperature, P2 is the final pressure, and T2 is the final temperature.
Substituting the given values, we get:
P1 = 106 kPa
T1 = 47°C + 273.15 = 320.15 K
T2 = 77°C + 273.15 = 350.15 K
So, P2/T2 = P1/T1
P2 = P1 × (T2 / T1)
P2 = 106 kPa × (350.15 K / 320.15 K) = 115.44 kPa
Therefore, the new pressure of the hydrogen gas will be 115.44 kPa when it is heated to 77°C at constant volume.
please vote brainliest, have a great day :)
which of the following elements is the largest?
A. Boron
B. Nitrogen
C. Oxygen
D. Carbon
Answers
Answer:
A) Boron
Explanation:
I put oxygen and it was wrong lol. So, it said it was Boron
The chemical equation below represents an unbalanced chemical reaction:
Fe + 0, → Fe,o,
When the equation is balanced, what coefficient is needed for Fe2O3?
A
1
B
2
С
3
D
4
Answers
Answer:
2
Explanation:
a massive object can distort the light of more distant objects behind it through the phenomenon that we call .target 1 of 6 2. blank are defined as subatomic particles that have more mass than neutrinos but do not interact with 2 of 6 3. the of spiral galaxies provide strong evidence for the existence of dark 3 of 6 4. matter made from atoms, with nuclei consisting of protons and neutrons, represents what we call blank 4 of 6 5. models show that the of the universe is better-explained when we include the effects of dark matter along with the effects of luminous 5 of 6 6. matter consisting of particles that differ from those found in atoms is generally referred to as ____
Answers
1. Gravitational lensing is the phenomenon that we call a massive object that can distort the light of more distant objects behind it.
2. WIMPs (weakly interacting massive particles) are defined as subatomic particles that have more mass than neutrinos but do not interact with normal matter.
3. The rotation curves of spiral galaxies provide strong evidence for the existence of dark matter.
4. Baryonic matter made from atoms with nuclei consisting of protons and neutrons, represents what we call ordinary matter.
5. Models show that the evolution of the universe is better-explained when we include the effects of dark matter along with the effects of luminous matter.
6. Matter consisting of particles that differ from those found in atoms is generally referred to as exotic matter.
What is dark matter? Dark matter is a kind of matter that scientists assume to exist since it does not interact with light and cannot be seen through telescopes. Dark matter is believed to account for approximately 27% of the matter in the universe. Dark matter interacts gravitationally with visible matter and radiation, but it doesn't interact with electromagnetism, making it completely invisible to telescopes that observe electromagnetic radiation, such as radio waves, infrared light, visible light, ultraviolet light, X-rays, and gamma rays.
To know more about dark matter refer to:
https://brainly.com/question/28256017
#SPJ11
what happens if you try to move the atoms very close to each other?
Answers
when a standing sound wave is generated in an open-open tube, the pressure inside the tube is the atmospheric pressure when the displacement of air is
Answers
When a standing sound wave is generated in an open-open tube, the pressure inside the tube is equal to atmospheric pressure when the displacement of air is at its maximum or minimum.
In an open-open tube, both ends are open to the atmosphere, allowing air to move freely in and out, the tube supports a standing wave pattern with nodes and antinodes. Nodes are points of minimum displacement, and antinodes are points of maximum displacement. At the nodes, the air particles are not displaced from their equilibrium positions, leading to fluctuations in pressure. These pressure fluctuations result in maximum pressure differences from the atmospheric pressure.
On the other hand, at antinodes, the air particles have maximum displacement, and the pressure remains constant, equal to the atmospheric pressure. In summary, in a standing sound wave generated in an open-open tube, the pressure inside the tube is equal to the atmospheric pressure when the displacement of air is at its maximum or minimum. This occurs at the antinodes of the standing wave pattern, where the air particles have maximum displacement and experience no pressure fluctuations.
Learn more about antinodes at:
https://brainly.com/question/30640087
#SPJ11
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
Can somebody explain to me on how to do this (in the picture)? Thanks!

Answers
According to chemical equilibrium, the value of equilibrium constant is 1.33.
What is chemical equilibrium?Chemical equilibrium is defined as the condition which arises during the course of a reversible chemical reaction with no net change in amount of reactants and products.A reversible chemical reaction is the one wherein the products as soon as they are formed react together to produce back the reactants.
At equilibrium, the two opposing reactions which take place take place at equal rates and there is no net change in amount of the substances which are involved in the chemical reaction.At equilibrium, the reaction is considered to be complete . Conditions which are required for equilibrium are given by quantitative formulation.
The equilibrium constant is given as, K=[C]/[A][B]²=[0.760]/[0.290][1.40]²=1.33.
Thus, the value of equilibrium constant is 1.33.
Learn more about chemical equilibrium,here:
https://brainly.com/question/8983535
#SPJ1
A screw has _____ over the cylinder.
Answers
corkscrew-shaped ridge
What is a screw’s output?The screw’s applied force (output force) is always larger than the screw’s applied force (input force). As a result, a screw’s mechanical advantage is always larger than one.Screws are a type of basic machine. They have a thread, which is a corkscrew-shaped ridge wrapped around a cylinder. When driving in a screw, the head is particularly designed to allow a screwdriver or wrench to hold the screw.
Another sort of inclined plane is the screw. When an inclined plane wraps around a straight rod-like structure, a spirally grooved surface with a pointed end is generated. It serves to keep things together.
To learn more about screw to refer:
https://brainly.com/question/19552791
#SPJ1
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
acetanilide: put in the molecular formula of the ions responsible for peaks at 43 and 92 . use the following order: c, h, o, n and include a ' ' at the end. a formatting example for mass of 44 would be c2h4o
Answers
The ions responsible for the peaks at 43 and 92 in acetanilide are C6H5O- and C6H5CONH2+, respectively.
Acetanilide has a molecular formula of C8H9NO, which has a molecular weight of 135 g/mol. The peak at 43 is due to the loss of a C6H5O- ion from the molecule, resulting in a fragment with a mass of 92. The peak at 92 is due to the presence of the C6H5CONH2+ ion in the molecule. This ion is formed by the loss of a CH3CO- ion from the molecule, resulting in a fragment with a mass of 92. The mass spectrometry data can be used to identify the fragments produced during the fragmentation of acetanilide and aid in the determination of its molecular structure.
In summary, the ions responsible for the peaks at 43 and 92 in acetanilide are C6H5O- and C6H5CONH2+, respectively. The mass spectrometry data can be used to identify the fragments produced during the fragmentation of acetanilide and aid in the determination of its molecular structure.
To learn more about acetanilide, visit here:
https://brainly.com/question/28608131
#SPJ11
Complete and balance the molecular equation, including the phases, for the reaction of aqueous potassium sulfate, k2so4 , and aqueous strontium iodide, sri2.
Answers
The complete and balanced molecular equation is-
K₂SO₄(aq) + SrI₂(aq) → KI(aq)+ SrSO₄(s)
Both sides of the reaction must have an equal amount of atoms in each element to balance the equation.
What is a balanced chemical reaction?A balanced chemical reaction is one in which both the reactant and product sides of the reaction include an equal number of atoms from each of the constituent components. For the chemical equation to adhere to the Law of the conservation of mass, it must be balanced.An equation that has the same number of each type of atom on both sides of the arrow is said to represent a balanced chemical reaction. A chemical reaction is represented symbolically in writing by a chemical equation. The chemical(s) used as the reactant(s) are listed on the left and the chemical(s) used as the product(s) are listed on the right. A chemical reaction requires an equal number of atoms in the reactants and products because, according to the rule of conservation of mass, atoms cannot be generated or destroyed throughout the process.How to balance a molecular chemical equation?First of all, identify the most complicated or complex substances.If possible, start with that material and pick an element or elements that are present in only one reactant and one product. To get the same number of atoms of this element(s) on both sides, adjust the coefficients.If polyatomic ions are present on both sides of the chemical equation, they should be balanced as a whole.The remaining atoms are then balanced, typically ending with the substance that is the least complicated and, if necessary, employing fractional coefficients. In order to get whole numbers for the coefficients if a fractional coefficient was used, multiply both sides of the equation by the denominator.To learn more about Molecular equation click on-
https://brainly.com/question/1594155
#SPJ4
Balanced molecular equation is :
K₂SO₄(aq) + SrI₂(aq) → KI(aq)+ SrSO₄(s)
What is Balanced equation?
If both the reactants and the products of a chemical reaction have the same number of atoms and total charge, the equation for the reaction is said to be balanced. In other words, the mass and charge balances on both sides of the reaction are equal.The equation must be balanced so that each type of atom appears in equal amounts on both the left and right sides of the arrow. This is accomplished by altering the compounds' coefficients (numbers placed in front of compound formulas).In an imbalanced chemical equation, the reactants and products of a reaction are provided, but the amounts required to satisfy the conservation of mass are not given.Learn more about the balanced equation with the help of the given link:
https://brainly.com/question/12192253
#SPJ4
How many grams of O2 gas are in a 3700 mL container at a pressure of 775 mmHg at 33oC?
Answers
Answer:
4.8 grams
Explanation:
Use PV=nRT
P: 775 mmHg (divide by 760 mmHg to get atm) -> 1.02 atm
V: 3700 mL (divide by 1000 to get L) -> 3.7L
n: ?
R: (a constant) 0.0821L * atm/k *mol
T: 33 C (add 273 to get K) -> 306K
Move equation so n is on the side: PV/RT = n. Plug the numbers into the equation.
\(\frac{1.02atm * 3.7L }{306K} * \frac{K * mol}{0.0821L *atm} = 0.15 mol\)
Then, convert moles to grams using the molar mass of O2 which is 32g/mol.
\(0.15 mol * \frac{32g}{mol}\)= 4.8g
4.8g is the mass of oxygen gas which are in a 3700 mL container at a pressure of 775 mmHg at 33oC.
What is mass?A body's mass is an inherent quality. Prior to the discoveries of the atom as well as particle physics, it was widely considered to be tied to the amount of matter of a physical body. It was discovered that, despite having the same quantity of matter in theory, different atoms and elementary particles have varied masses.
There are various conceptions of mass in contemporary physics that are theoretically different but physically equivalent. The resistance of the body to accelerate (change of velocity) in the presence of a net force can be measured experimentally as mass.
PV=nRT
P= 775 mmHg/760 = 1.02 atm
V=3700 mL/1000 = 3.7L
n=
R=0.0821L× atm/k ×mol
T=33 C + 273=306K
1.02×3.7/0.0821×306= n
n=0.15 moles
mass = 0.15×32=4.8g
Therefore, 4.8g is the mass of oxygen.
To know more about mass, here:
https://brainly.com/question/19694949
#SPJ2