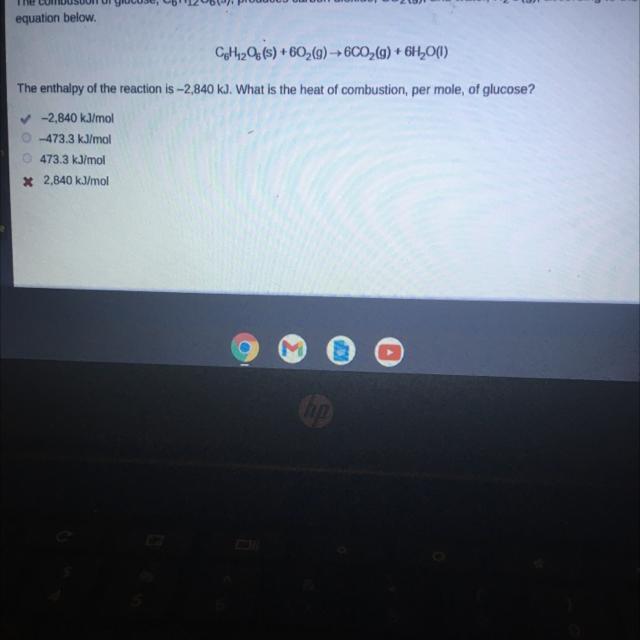
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according to the
equation below.
CoH1206(s) +602(g) → 6CO2(g) + 6H2O(1)
The enthalpy of the reaction is -2,840 kJ. What is the heat of combustion, per mole, of glucose?
✓-2,840 kJ/mol
473.3 kJ/mol
473.3 kJ/mol
* 2,840 kJ/mol
See
Answers
Answer:
A would be the correct answer
Explanation:
look at the pic
Related Questions
Calculate the number of grams of solute in 1.000 L of 0.943 M potassium iodate
Answers
The number of grams of solute in 1.000 L of 0.943 M potassium iodate is 201.803g
0.943M means, 1L contains 0.943 moles KIO3.
Amount in g = no of moles× molar mass.
Molar mass of KIO3 = 214 g/mol.
So, mass of KIO3 present= 214×0.943 g = 201.803g
What are solute?A substance dissolved in a solution is called a solute. In liquid solutions, the amount of solvent is greater than the amount of solute. One of the best examples of a solute in our daily activities is salt and water. Salt dissolves in water and therefore salt is a solute.
The major types of solute are:
GaseousLiquidSolidTo learn more about solute, refer;
https://brainly.com/question/7932885
#SPJ9
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
What was earth’s surface like? Landmasses? First land plants
Answers
Answer:
During the early Paleozoic Era, the Earth's surface was very different from what it is today. The continents were arranged differently, forming one large supercontinent called Pangea. This landmass was surrounded by a single large ocean called Panthalassa. The climate was much warmer and wetter than it is today, with no ice caps at the poles.
The first land plants, known as bryophytes, appeared during the early Silurian Period, around 430 million years ago. These plants were small and simple, lacking roots and vascular tissue. They grew in damp environments, such as along the edges of lakes and streams. They were important in the development of soils and in the colonization of land by other organisms, such as insects and other arthropods.
Calculate the mass percent of Cl in SiCl2I2.
Answers
The mass percent of Cl in SiCl2I2 is 20.13%.
The chemical formula of SiCl2I2 can be broken down into its constituent elements, Si, Cl, and I. The total mass of the compound is the sum of the masses of these elements. Then, we can find the mass percentage of chlorine in SiCl2I2.
The mass of Si is 28.09 g/mol, the mass of Cl is 35.45 g/mol, and the mass of I is 126.9 g/mol. Therefore, the molar mass of SiCl2I2 is:
Molar mass of SiCl2I2 = (28.09 g/mol) + 2(35.45 g/mol) + 2(126.9 g/mol)
= 352.79 g/mol
To find the mass percentage of chlorine in SiCl2I2, we need to determine the mass of chlorine in the compound. There are two chlorine atoms in the molecule, so the mass of chlorine is:
Mass of Cl = 2(35.45 g/mol) = 70.9 g/mol
Now, we can calculate the mass percentage of Cl in SiCl2I2:
Mass percentage of Cl = (Mass of Cl / Molar mass of SiCl2I2) × 100%
= (70.9 g/mol / 352.79 g/mol) × 100%
= 20.13%
for more questions on mass
https://brainly.com/question/24191825
#SPJ8
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
Write the Ka expression for an aqueous solution of hypochlorous acid: (Note that either the numerator or denominator may contain more than one chemical species. Enter the complete numerator in the top box and the complete denominator in the bottom box. Remember to write the hydronium ion out as , and not as )
Answers
Answer: The Ka expression for an aqueous solution of hypochlorous acid is \(K_{a} = \frac{[H_{3}O^{+}][OCl^{-}]}{[HClO]}\).
Explanation:
The chemical formula of hypochlorous acid is HClO. So, when it is added to water (solvent) then its dissociation is as follows.
\(HClO + H_{2}O \rightarrow H_{3}O^{+} + Cl^{-}\)
When we write the equilibrium constant for this reaction then it is called acid acid dissociated constant.
Hence, the expression for acid dissociation constant of this reaction is as follows.
\(K_{a} = \frac{[H_{3}O^{+}][OCl^{-}]}{[HClO]}\)
Thus, we can conclude that the Ka expression for an aqueous solution of hypochlorous acid is \(K_{a} = \frac{[H_{3}O^{+}][OCl^{-}]}{[HClO]}\).
How many moles are there in 80 grams of oxygen gas (O2)? *
Answers
Answer:
The number of moles in 80 g of O2 = 3280 = 2.5.
Explanation:
Trust me ._.
what is the circulatory do
Answers
The circulatory system delivers oxygen and nutrients to cells and takes away wastes
Answer:
The circulatory system delivers oxygen and nutrients to cells and takes away wastes.
Explanation:
if it is about science its correct :)
PLSSSSS I NEED HELP MY AMPLIFY SIM WON’T WORK I DONT KNOW WHAT TO DO FOR AMPLIFY TAB 3.5.!! IM IN THE PURPLE GROUP !! The lead chemist wants you to determine what is happening to the freedom of movement of an object’s molecules when you smell something. Is it possible to smell a chocolate bar when it is a solid? Launch the Sim and investigate.
Use the Sim to determine if the molecules of a substance can be in two different phases at the same time.
Go through each substance and see if you can get it to exist in two phases at once.
Record as much evidence as you can in the table below.
Answers
When a chocolate bar is solid, it is able to smell it. It is also important to note that a substance 's molecules cannot be in two distinct phases at the a time.
Why is this the case ?In general, it is not possible for a the molecules of the chocolate to be in two distinct phases at the same time.
It must be noted that smelling a solid, such as a chocolate bar, however, mean the release of loose molecules from the solid, which may then move through the air and reach our olfactory receptors in our nose, allowing us to sense the fragrance.
Learn more about phase transition:
https://brainly.com/question/29795670
#SPJ1
You have two containers at 0°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L of oxygen gas.
Which statement is true?
-))
A)
Both containers contain 6.022x1023 molecules of gas.
B)
B)
The hydrogen gas container has more molecules than the oxygen gas
container
The oxygen gas container has more molecules than the hydrogen gas
container
D)
Both containers have the same number of molecules.
Answers
Answer:
D
Explanation:
According to Avogadro's law, equal volumes of different gases at the same temperature and pressure have equal number of molecules.
It cannot however be a because according to ideal gas equation the number of moles in each container is 0.999moles which translates to 6.019*10^23 molecules
Answer:
Both containers have the same number of molecules.
Explanation:
Cu+2 + Cl-1. Help me plssss
Answers
Answer:
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl.
Explanation:
of all the hydrogen nuclei in the ocean, 0.0156 how much deuterium could be obtained from 1.0 gal of ordinary tap water
Answers
Answer:
Poop Butt.
Explanation: Poop Butt.
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2
How many liters of propane gas (C3H8) will undergo complete combustion with 37.0 L of oxygen gas? (20pts) C3H8(g) + 5O2(g) ⇨ 3CO2(g) + 4H2O(g)
Answers
Since we are working with gases, the liters can be taken as moles.
According to the given balanced reaction, 1 liter of propane reacts completely with 5 liters of oxygen, use this ratio to find the liters needed to react with 37 liters of oxygen:
\(37LO_2\cdot\frac{1LC_3H_8}{5LO_2}=7.4LC_3H_8\)It means that 7.4 liters of propane will be needed.
Imagine an element, X, that has two naturally occurring
isotopes. If you know the mass and the percentage
abundance of one of the isotopes, how would you
determine the percent abundance of the other isotope?
Describe your problem-solving process
Answers
The characteristics of the percentage allow finding that the abundance of the second isotope is
b = 100 - a%
Chemical elements are stable structures that appear in the periodic table, these structures can have some variations, changing the number of protons changes the type of chemical element, but changing the number of neutrons, with the same atomic number, the elements with all their hardware properties, this type of atoms is called isotopes.
In this case it is said that the element X has two isotopes, if the abundance of one of them is a, the sum of all the abundances must give the total value of 1 or 100%,
the total abundance is
1 = a + b
b = 1- a
this is the abundance of the other isotope, if you prefer it can be given as a percentage
b = 100 - a%
In conclusion using the characteristics of the percentage we can find the abundance of the second isotope is
b = 100 - a%
learn more about percentage here:
https://brainly.com/question/21814361
photo attached
if anyone from my class sees this no you didn't

Answers
Answer:
I think its b or c
Explanation:
hope this helps I mainly think b
HELPPP ANSWERSSS !!!!!
A.
10
B.
9
C.
7
D.
18

Answers
A small amount of chemical splashes in Frank’s eye. What should Frank do immediately?
Answers
Answer:
A small amount of chemical splashes in Frank's eye. What should happen next? Frank should go to the eyewash station while his lab partner tells the teacher what happened.
Explanation:
Brainlist
Eddie dropped a baseball off the roof of his building. The circle graphs below show the ball's energy distribution as it falls. In the
graphs, the baseball's potential energy is shown relative to ground level, and the baseball's thermal energy is shown relative to its
thermal energy before it was dropped.
Put the graphs in order to show the ball's energy distribution as it falls.
Kinetic Energy
Potential Energy
Thermal Energy
Y’all what’s the order

Answers
The order of the graphs that shows the movement of the object is; B C E A D.
What is the energy?We know that energy has to do with the ability to do work. In this case, we can see that the object has been dropped from a height and we must have in mind the principle of the conservation of mechanical energy. In this principle, it has been stated that energy can neither be created nor destroyed but can be converted from one form to another.
As the object is falling, there are three main kinds of energy that would come into play and these are; mechanical energy, kinetic energy and potential energy.
The kinetic energy is the energy that is in motion while the kinetic energy has to do with the energy that is at a point. Looking at the graph, we know that the amount of the thermal energy would increase the farther the object falls to the ground. Let the letters be shown as A B C D E standing for each of the graphs.
Learn more about energy:https://brainly.com/question/1932868
#SPJ1
Choose the option that would convert mg/L
into molarity (mol/L).
A. Convert mg/L to get g/L then multiply by the volume (L).
B Convert mg/L to g/L then divide by molar
mass (g/mol).
C. Convert mg/L to g/L then divide by the volume (L).
D. Convert mg/L to g/L then divide by the
number of moles (mol).
Answers
The correct option that would convert mg/L into molarity (mol/L)- Convert mg/L to get g/L then multiply by the volume (L). so, option (a) is correct.
What is molarity ?
The amount of solute in one mole per liter of solution is known as molarity. For instance, when table salt is dissolved in water, the solute is salt, and the solution is water. 58.44 grams make up one mole of sodium chloride. One molar solution, often known as 1M, is created when 58.44 grams of sodium chloride are dissolved in one liter of water.
What is volume ?
Volume, a three-dimensional quantity, is used to calculate the capacity of a solid shape. It suggests that the volume of a closed figure determines the amount of three-dimensional space it can occupy.
Therefore, the correct option that would convert mg/L into molarity (mol/L)- Convert mg/L to get g/L then multiply by the volume (L). so, option (a) is correct.
Learn more about molarity from the given link.
https://brainly.com/question/26873446
#SPJ1
Answer: Not A, its B
Explanation: B
Determine whether each of these reactions occur through an SN1 , SN2 , E1, or E2 mechanism. A. A cyclohexane ring with a bromine substituent is treated with sodium tert butoxide in tert butanol. The product is cyclohexene and sodium bromide. A. The mechanism of Reaction A is: SN1 SN2 E1 E2
Answers
Answer:
E2
Explanation:
We can generally regard the bromocyclohexane as having a secondary carbon atom to Which the bromine atom is attached.
Tert-butoxide in tert-butanol favours the formation of an alkene (elimination) leading to the predominance of the less substituted alkene.
Generally, bulky bases such as tert-butoxide favours elimination by E2 mechanism over substitution.
The mechanism of Reaction A is E2 and also mechanism over substitution.
What is Bromine substituent?
We can generally regard the Bromo cyclohexane as maintaining a secondary carbon atom to Which the bromine atom is connected.
Tert-butoxide in tert-butanol prefers the formation of an alkene (elimination) leading to the predominance of the undersized substituted alkene. Therefore, Commonly, bulky bases such as tert-butoxide favors elimination by the E2 mechanism over substitution.
Find more information about Bromine substituent here:
https://brainly.com/question/23192856
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
Answer please I give lots of points

Answers
Answer:
4
Explanation:
Calculate the relative atomic mass of M

Answers
Answer:
63.55
Explanation:
relative atomic mass=(mass of isotope1×relative abundance)+(mass of isotope 2×relative abundance)/100
r.a.m=(62.93×69.09)+(64.93×30.91)/100
=(4347.8337)+(2006.9863)/100
=6354.82/100
=63.55
What is a quasar?
Two stars moving around each other.
A star that emits a repeated radio signal.
A star that emits intense radio and light energy.
A system of stars held together by gravity.
A collapsed star emitting no light.
Answers
A quasar is a star that emits intense radio and light energy.
A quasar is an extremely bright radio source. It is called a quasi-stellar radio source. It appears to be like a star but is not a star. These quasars are young galaxies that are located far away from us and are highly luminous. The luminosity of a quasar is 1000 times greater than the luminosity of a milky way galaxy.
A quasar is powered by a supermassive black hole with its mass ranging from millions to tens of billions of solar masses, surrounded by a gaseous accretion disc.
The quasars were first discovered in the 1950s using the Hubble space telescope and were found to be a massive bright source emitting radio waves of unknown origin. But now, millions of quasars were discovered.
To know more about stars, click below:
https://brainly.com/question/1041175
#SPJ1
calculate the number of molecules in 6.4g of Sulphur (iv) oxide
Answers
Answer:
N= mass/molar mass
complete the solve
Do step 3 as outlined in the lab guide. Record your results in the appropriate blanks.
A =
B =
C =
D =
E =
F =
G =
H =
Answers
Some tips to follow when doing lab practical are:
Avoid parallax errorsRecord your observations and data accuratelyUse the appropriate lab equipment.How do we know?From the table, Column 1 represents the time in half-life cycles, ranging from the initial state to 8 cycles. Column 2 shows the predicted number of radioactive atoms at each time point, based on the assumption that the number of atoms reduces by half in each half-life cycle.
Column 3 represents the simulated number of radioactive atoms at each time point and corresponds to the predicted values of the simulation.
In conclusion, the results as outlined in the lab guide are A= 27 B= 16 C= 9 D= 4 E= 2 F= 2 G= 0 H= 0.
Learn more about half-life cycles at:
https://brainly.com/question/15976750
#SPJ1
#complete question:
Do step 3 as outlined in the lab guide. Record your results in the appropriate blanks. A = B = C = D = E = F = G = H = A 3-column table with 9 rows. Column 1 is labeled Time half-life cycles, n with entries Initial, 1, 2, 3, 4, 5, 6, 7, 8. Column 2 is labeled Predicted radioactive atoms with entries 100, 50, 25, 13, 6, 3, 2, 1, 0. Column 3 is labeled Simulated radioactive atoms with entries 100, A, B, C, D, E, F, G, H.
Which state of matter takes the shape but NOT the volume of its container?
solids
liquids
gases
Both B & C
Answers
Answer:
solids and liquids
Explanation:
Answer:
liquid is the state of matter that has a definite volume but takes the shape of its container
I need help with the following question

Answers
The right answer is (KP)-1/2. Option A.
An expression of the equilibrium constant in terms of partial pressure is denoted as Kp. The equilibrium constant Kp is equal to the partial pressure of the product divided by the partial pressure of the reactant, and the partial pressure increases with a force equal to the coefficient of the substance in the equilibrium equation.
Kp is the equilibrium constant used to express equilibrium concentrations at atmospheric pressure and Kc is the equilibrium constant used to express equilibrium concentrations in molar terms. The relationship between Kp and Kc depends on the change in the number of moles of gaseous reactants and products. Kp has exactly the same form as Kc but partial pressure is used instead of concentration. Gases to the right of the formula are at the top of the print, and gases to the left are at the bottom.
Learn more about The equilibrium constant here:-https://brainly.com/question/3159758
#SPJ1