Answers
Answer:
All of the elements in group 17 all have 7 valence electrons. This is one thing they all share in common.
Explanation:
Related Questions
Why are indicators used in titrations? *
O Indicators are used to show when a solution is basic.
O Indicators are used to show when a solution is acidic.
O Indicators are used to show when a solution is neutral.
O Indicators are not used in titrations.
0.00r

Answers
A titration's end can be indicated by an indicator because a significant pH change happens close to the standard solution of acid-base titrations. For acid-base titrations, use an indicator.
What's the proper name for an acid-base titration?Acid-base titration is the term for a procedure that uses an acid-base reaction. It is known as a redox titration whenever a reduction process is used. Quantitative chemical analysis is a sort of titration, commonly referred to as volumetric analysis.
In titration, why are indicators used?To determine the equivalency or the point in a reaction where a pH change occurs, indicators are therefore utilized in titrations. As an additional indicator that changes the color when pH changes, methylene yellow is also employed. In acidic solution, it is colored red, whereas in basic solution, it is colored yellow.
To know more about acid-base titration visit:
https://brainly.com/question/2728613
#SPJ1
As you move from left to right across the periodic table, electronegativity
Answers
Answer:
Electronegativity increases as you move across the periodic table from left to right.
Explanation:
Answer:
increases
Explanation:
PLEASE HELPPP
UNIT TEST Chemical Reactions - Part 1 Which equation is balanced? CP+20 - PO₂ 2P+ 0; --P.₂O. 4P + 50., --2P.0. 4P - 20. - 27.0. - →
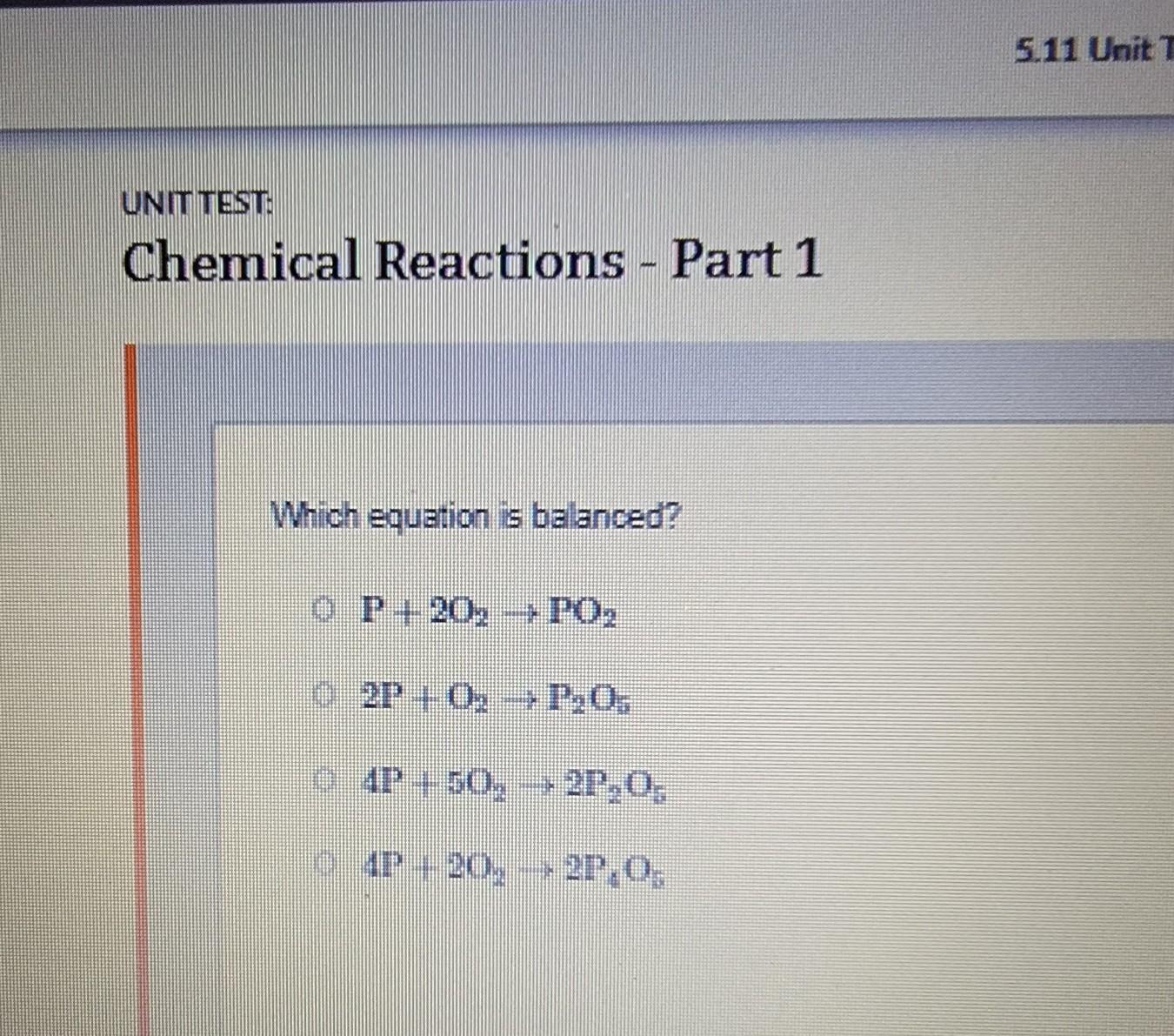
Answers
Answer:
2P+ 02 → P205Explanation:
I Thinks it B!!!
but please don't take my word i'm not really good at math.What happens to the pH when a a small amount of acid is added to a buffered solution?
A.the pH goes up to 14.
B.The pH goes down to 1.
C.The pH stays about the same.
D.The pH goes to 7.
Answers
C. The pH stays about the same.
A buffered solution resists changes in pH upon addition of small amounts of acid or base. The buffer system in the solution will react with the added acid, keeping the pH relatively constantAnswer:
C.The pH stays about the same.
Explanation:
Buffer reactions maintain stable pH of solutions.
Ammonium nitrate (NH4NO3) is a common component of fertilizer because it is a great source of two nitrogen-containing ions that easily dissolve into water. A large batch of this liquid fertilizer is made by adding 2.00 pounds of ammonium nitrate to 35.00 gallons of water. What is the molarity of this solution? (Hint: 1 lb. = 454 g and 1 gal. = 3.79 L.)
Answers
The molarity of the ammonium nitrate solution is 2281 M.
What is molarity?Molarity is defined as the number of moles of solute per liter of solution. It is also known as molar concentration of the solution and is used to calculate amount of substances in the solution.
Molarity = n/M
n = m / MW
m = 2 lbs = 1000/2.2 = 909 g
V = 53 x 3.79/1 = 200.9
MW = 80.04 g
M = m/Mw / V
M = 909/80.04 /200.9
M = 2281 M
Thus, the molarity of the ammonium nitrate solution is 2281 M.
To learn more about molarity, refer to the link below:
https://brainly.com/question/8732513
#SPJ1
An atom has atomic number 5 and mass number 11. How many neutrons does the atom have?
A- 5
B- 6
C- 10
D- 11
Answers
Answer:
B
Explanation:
neutrons + atomic number/proton/electron for a neutral atom = mass number
e.g
mass number of Chlorine 35.5
proton number 17
neutron number will be 35.5-17 = 18.5
Therefore.....
your question deduced says
atomic number is 5
mass number is 11
neutron number is however mass number minus atomic number 11-5 =6
how does cohesion affect the evaporation rate of water
Answers
Answer:
Evaporation occurs because among the molecules near the surface of the liquid there are always some with enough heat energy to overcome the cohesion of their neighbors and escape. At higher temperatures the number of energetic molecules is greater, and evaporation is more rapid.
What ways is carbon added to the atmosphere
Answers
Answer:
One way is through fossil fuels. When people burn fossil fuels it enters the atmosphere as carbon dioxide gas. Another way is through animals. Most animals exhale carbon dioxide as a waste product which gets into the atmosphere.
Balance the equations by putting the necessary coefficients in the blanks. Normally we do not write 1s when balancing, but for this particular question you need to include them for full credit. __Na3N___ Na +__ N2 ___H3PO4 + __ KOH __K3PO4 + __ H2O __ N2 +__ H2 __ NH3 __H2O2 __ O2 + __ H2O __ Zn + __ HCl __ ZnCl2 + __H2 __ C2H6 + __ O2 __ CO2 + __H2O __ CuCl2 + __H2S __ CuS + __HCl

Answers
Balancing a chemical equation is the process of ensuring that the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products.
Balance the chemical eqations given in the problem?
Na3N → 3 Na + ½ N2H3PO4 + 3 KOH → K3PO4 + 3 H2ON2 + 3 H2 → 2 NH3H2O2 → O2 + 2 H2OZn + 2 HCl → ZnCl2 + H2C2H6 + 7/2 O2 → 2 CO2 + 3 H2OCuCl2 + H2S → CuS + 2 HClChemical equations are used to describe the reactants and products in a chemical reaction. These equations are written using chemical formulas and symbols, indicating the types and numbers of atoms or molecules involved in the reaction. However, these equations must be balanced to obey the law of conservation of mass, which states that the total mass of the reactants must equal the total mass.
To learn more about chemical equation, visit: https://brainly.com/question/29886207
#SPJ1
Alkanes, alkenes, and alkynes are all hydrocarbons. What is one difference between alkanes and alkenes?A) Alkenes form a branched chain; alkanes form only straight chains.B) Alkenes are saturated hydrocarbons; alkanes are unsaturated hydrocarbons.C The characteristic functional group of an alkene is the carbon-carbon double bond.D) The characteristic functional group of an alkene is the carbon-carbon triple bond.
Answers
Answer
C. The characteristic functional group of an alkene is the carbon-carbon double bond.
Explanation
Why does ear escape from a tire when a tire valve is opened
Answers
Air escape from a tire when tire valve is opened because the pressure from the weight of the car is forcing the already pressurized air out .
Why does air escape from tire when tire valve is opened?When air is filled in tires, it get into more compact area than the outer atmosphere, therefore particles in tire are close to each other and exert pressure continuously on tire walls to get out of it. Thus, material of the tire need to be pressure resistant as much possible.
After opening the tire valve, air starts leaving with huge sound as strain outside the tire is weaker than that inside the tire. Molecules strike with the same force on larger area and pressure starts diminishing.
To know more about tire valve, refer
https://brainly.com/question/3720473
#SPJ1
Note: The question given on the portal is incomplete. Here is the complete question.
Question: Air leaves a tire when the tire valve is opened because
A. the pressure outside the tire is lower than the pressure inside the tire.
B. the pressure outside the tire is greater than the pressure inside the tire.
C. the temperature is higher outside the tire than inside the tire.
D. there are more gas particles outside the tire than inside the tire.
What is the mass of a rectangular piece of copper 24.4cm x 11.4 cm x 7.9 cm? The density of copper is 8.92g/cm3.
Answers
The mass of the rectangular piece of copper is 18,869 g (approx).In conclusion, the mass of a rectangular piece of copper with dimensions 24.4cm x 11.4 cm x 7.9 cm and a density of 8.92 g/cm³ is 18,869 g (approx.).
The given dimensions of the rectangular piece of copper are:Length = 24.4 cmWidth = 11.4 cmHeight = 7.9 cmThe formula to calculate the mass of an object is given by;
Mass = Density x Volume
Here, the density of copper is given as 8.92 g/cm³.
Therefore, the first step is to calculate the volume of the rectangular piece of copper.The formula to calculate the volume of a rectangular object is given by:
Volume = Length x Width x Height
So,Volume = 24.4 cm x 11.4 cm x 7.9 cm= 2115.432 cm³Now we will use the mass formula:
Mass = Density x Volume= 8.92 g/cm³ x 2115.432 cm³= 18,869.27824 g= 18,869 g (approx.)
For more such questions on copper
https://brainly.com/question/29176517
#SPJ8
What is the IUPAC name for CH3CH(OH)CH(CH3)CH(CH3)2 ?
Answers
The IUPAC name of the following structural formula CH3CH OH−CH3 is propan-2-ol.
What is the structural formula?
The molecular structure of a chemical compound is graphically represented by the structural formula of the complex, which demonstrates how the atoms may be arranged in three-dimensional space. Structural formulas offer a more comprehensive geometric depiction of the molecular structure than other forms ofof chemical formulas, which have fewer symbols and less descriptive power. For instance, many chemical compounds exist in many isomeric forms that have the same molecular formula but different enantiomeric structures..To know more about structural formula, click the link given below:
https://brainly.com/question/14611418
#SPJ1
Hello, this is a science task and it is due today I would really appreciate it if you guys can help me answer and explain what is the difference between expansion and contraction:) I will mark brainliest!

Answers
Answer:
Expansion: increase in size Contraction: decrease in size
Explanation:
The increase in size of an object on heating is called expansion where as the decrease in size of an object on cooling is called contraction.
How much heat capacity, in joules and in calories, must be added to a 75.0-g iron block with a specific heat of 0.499J/g °C to increase its temperature from 25 °C to its melting temperature of 1535 °C?
Answers
Answer:
56511.75 J
13506.3 Calories
Explanation:
Applying,
Q = cm(t₂-t₁).................. Equation 1
Where Q = amount of heat, m = mass of the iron, c = specific heat capacity of the iron, t₁ = initial temperature, t₂ = final temperature.
From the question,
Given: m = 75 g, c = 0.499 J/g.°C, t₂ = 1535°C, t₁ = 25°C
Substitute these values into equation 1
Q = 75(0.499)(1535-25)
Q = 75(0.499)(1510)
Q = 56511.75 J
Q in Calories is
Q = (56511.75×0.239)
Q = 13506.3 Calories
What happens to the temperature of water as it is boiling? Increase, decrease, or remains the same?
Answers
Answer:
Increased
Explanation:
Identify the correct structure of 5-bromo-4-isopropylheptanoic acid.
Answers
Answer:
See attached picture.
Explanation:
Hello,
In this case, given the IUPAC name, we can infer we have a seven-carbon carboxylic acid that has a bromine at the fifth carbon, an isopropyl at the fourth carbon and the carboxyl functional group (COOH) at the first carbon, thus, on the attached document, you will find the correct structure.
Best regards.

Select the correct image.
Which structure is a valid representation of a hydrocarbon molecule?
Н
1
Н-С-Н.
Н
Н
Н
Н
Н
1
н-с-н
Н Н
Н Н
1 1
1 1
Н-С—С—С—С—С-Н
1 1 1 1
Н Н H Н Н
H— С—С=С—С—С-Н
1
1 І
1
Н Н Н Н Н
Н
1
H-C-H
Н Н
Н Н
1 1
Н-С—С—С С— С
1
1
1
Н Н Н Н Н
Н
1
H-C-H
Н
Н
1
1
H-C— C-CEC— C-H
Н н н н Н
Answers
Answer:
H-C-H
Explanation:
hydrogen plus carbon hdrocloide
If the mass of a helicopter is 4500 kg and the net force on it is 18000 n, what is the helicopters acceleration
Answers
Answer:
The answer is
4 m/s²Explanation:
The force of an object can be found by using the formula
F = m × awhere
m is the mass
a is the acceleration
F is the force
Since we are finding the acceleration
\(a = \frac{F}{m} \)
From the question
F = 18000 N
m = 4500 kg
Substitute the values into the above formula and solve
That's
\(a = \frac{18000}{4500} \\ = \frac{180}{45} \)
We have the final answer as
4 m/s²Hope this helps you
Which of the following is an example of freshwater? a Coral Reefs b Rivers c Tide pools
Answers
Answer:
rivers
Explanation:
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
The pOH of a 0.310 M solution of NaOH is
Answers
The pOH of a 0.310 M solution of NaOH is 0.5086. The concentration of a solution's hydroxide ion (OH-) is measured by pOH.
Elaborating:
NaOH is strong base, ދ [OH-] = 0.310 M
рон = - 20g [011]
= - Log [0.310]
0.5086
port of the solution is 0.5086
What is pOH and how does it work?pOH is the basis for estimating the hydroxide particle fixation in the arrangement. It is used to determine the solution's simplicity. The negative logarithm of the concentration of hydroxide ions is equivalent to pOH. pOH = – log [OH–]
What Is the Meaning of pOH?The concentration of a solution's hydroxide ion (OH-) is measured by pOH. All things considered, it tends to be utilized as a mark of a substance's alkalinity or even its electrical conductivity at times.
What are weak acids and pH pOH?A logarithmic measurement of the concentration of hydroxide ions (OH-) in a solution is referred to as pOH. A pH scale that ranges from 0 to 14 is used to measure pH. The higher the concentration of H+ ions in a solution and the more acidic it is, the lower the pH.
Learn more about pOH :
brainly.com/question/27526649
#SPJ1
Which of these statements is most likely to be part of a safety contract?
A. Loose clothing and jewelry are permitted in the lab as long as no toxic chemicals are being used.
B. Safety goggles must be worn during all laboratory activities.
C. An emergency eyewash station should be set up next to each lab table before each lab session.
Answer: It's B
Answers
Answer: B. Safety goggles must be worn during all laboratory activities.

3. 7.5 g of hydrochloric acid react with 6.5g of zinc to produce
hydrogen gas and zinc chloride. Write the equation below,
balance it, and then determine the grams of hydrogen gas
produced by each reactant,

Answers
0.4141 grams of hydrogen gas will be produced from the reaction.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and zinc (Zn) can be written as follows:
2 HCl + Zn → ZnCl2 + H2
To determine the grams of hydrogen gas produced by each reactant, we need to use the concept of stoichiometry. First, we calculate the number of moles of each reactant.
The molar mass of HCl is approximately 36.5 g/mol, and the molar mass of Zn is approximately 65.4 g/mol.
For hydrochloric acid (HCl):
Number of moles = Mass / Molar mass = 7.5 g / 36.5 g/mol = 0.205 moles
For zinc (Zn):
Number of moles = Mass / Molar mass = 6.5 g / 65.4 g/mol = 0.0993 moles
According to the balanced equation, the mole ratio between HCl and H2 is 2:1. Therefore, for 0.205 moles of HCl, the theoretical yield of H2 is 0.205 moles.
The molar mass of hydrogen gas (H2) is approximately 2.02 g/mol.
To determine the mass of hydrogen gas produced:
Mass of H2 = Number of moles of H2 × Molar mass of H2
= 0.205 moles × 2.02 g/mol
= 0.4141 g
Therefore, 0.4141 grams of hydrogen gas will be produced from the reaction.
for more questions on hydrogen
https://brainly.com/question/24433860
#SPJ8
1. A rocket can be powered by the given reaction: N₂O4(1)+2N2H4(1)→ 3N2(g) + 4H₂O(g). An engineer designed the rocket to hold 1.00 kg of N₂O4 and excess N₂H4. [N 14.007 amu; H 1.008 amu; O 15.999 amu] (1000 g = 1kg) a) Given the balanced chemical equation above, what is the mole relationship between N₂O4 and N₂ ? (3 pts) N₂O +z M >N₂) 1/ b) How much N₂ would be produced with the 750 g N₂O4? (10 pts) c) If 650 g of N₂ was obtained, what is the percent yield?
Show work please
Answers
According to the balanced reaction, one mole of N₂O₄ produce 3 moles of N₂. Thus 750 g of N₂O₄will produce 684.7 g of N₂. If the actual yield of N₂ is 650 g, the percent yield is 94.9 %.
What is percent yield?The percent yield of a reaction is the ratio of actual yield to the theoretical yield multiplied by 100.
According to the given balanced reaction, one mole of N₂O₄ produce 3 moles of N₂. The molar mass of N₂O₄ is 92 g/mol and the molar mass of 3 moles of N₂ is 84 g. Thus 92 g of N₂O₄ produce 84 g of N₂.
The mass of N₂ produced from 750 g of N₂O₄ is then calculated as follows:
mass of N₂ = ( 750 × 84 /92)
= 684.7 g.
The actual yield of N₂ is given 650 g. Thus the percent yield of N₂ is calculated as:
Percent yield = (650 / 684.7) × 100
= 94.9 %.
Hence, the amount of N₂ produced from 750 g of N₂O₄ is 684.7 g and the percent yield is 94.9%.
To refer more about percent yield find the link here:
https://brainly.com/question/12704041
#SPJ1
N2 + H2 -> NH3
How many moles of H2 gas are used to make 36.5 grams of NH3?
Answers
Explanation:
First, you need to balance the equation:
N2 + 3 H2 ====> 2 NH3
so three moles of H2 result in 2 moles of NH3
ratio of 3:2
How many moles of NH3 is 36.5 gm ??
Using periodic table NH3 mole weight = 14.007 + 3*1.008 =17.031 g/mole
36.5 g / 17.031 g/mole = 2.14 moles of NH3
Using the ratios above 3/2 = x / 2.14 shows x = 3.21 moles of H2 needed
True or False?
1. Water does not control Earth’s temperatures because the maximum amount of water vapor in the atmosphere depends on the air temperature.
2. Oxygen is a minor component of the atmosphere but a major contributor to global warming.
3. Greenhouse gases consist of molecules with 3 or more atoms
4. Greenhouse gases with asymmetric vibrations DO NOT absorb infrared energy
5. Ozone is a naturally occurring gas (O3) that absorbs UV rays, preventing them from reaching Earth's surface
6. Increasing concentrations of carbon dioxide are responsible for rising global temperatures
7. Preventing pollution at its source is harder than remediating a polluted ecosystem
8. Greenhouse gases vary in their ability to trap heat.
9. Water vapor is the most abundant greenhouse gas, accounting for over half of the warming of all the greenhouse gases.
10. Decreasing amounts of algal bloom kills plants below the water surface
11. Some naturally occurring atmospheric gases, such as carbon dioxide and methane, help hold in warmth radiating off the surface of Earth.
12. Fracking is a safe method of oil and natural gas extraction.
Answers
1. Water controls Earth’s temperatures so the statement is false.
2. Oxygen is not a minor component of the atmosphere. the statement is False.
3. Greenhouse gases consist of molecules with 3 or more atoms. It is true.
4. Greenhouse gases with asymmetric vibrations absorb infrared energy. False.
5. . Ozone is a naturally occurring gas (O3) that absorbs UV rays, preventing them from reaching Earth's surface. True
6. Increasing concentrations of carbon dioxide are responsible for rising global temperatures. True.
7. False, preventing pollution at is source is not harder.
8. It is true that green house gases vary in their ability to trap heat.
9. It is true.
10. Decreasing amounts of algal bloom kills plants below the water surface. it is False.
11. The statement is true.
12. It is is false.
Detailed ExplanationGreenhouse gases are gases that trap heat resulting to what is known as green house effects and contributing greatly to global warming. Examples include methane, carbon dioxide etc.
Water control Earth’s temperatures because it brings about cooling.
when there's precipitation, the surfac of the earth becomes cooler.
Learn more on Greenhouse gases on https://brainly.com/question/12684997
#SPJ1
1. What is the Kinetic Energy of a 150 kg object that is moving with a speed of 15 m/s?
Answers
Answer:
16875 J
Explanation:
KINETIC ENERGY EQUATION = 1/2 m v^2
= 1/2 times mass times velocity of metres per second^2 (speed)
= 1/2 times 150 times by 15^2
= 16875 J (joules)
which statement about the sun is true?
A.The sun is a red giant
B. The sun will become a red giant
C. The sun began as a red giant
D. The sun cannnot become a reg giant
Answers
While folding laundry you notice that the socks are sticking to the T-shirts. What could explain this observation?
Both objects are uncharged.
The two objects are positively charged.
They are negatively charged objects.
They are oppositely charged objects.
Answers
Answer:
They are oppositely charged objects.
Explanation: