The balanced equation below shows the products that are formed when butane (C4H10) is combusted. 2C4H10 13O2 Right arrow. 8CO2 10H2O What is the mole ratio of butane to carbon dioxide? 1:4 1:5 13:8 13:10.
Answers
The mole ratio of butane to carbon dioxide in the equation is 1:4.
MOLE RATIO:Mole ratio is the proportion of one element/compound to another element/compound in a chemical reaction.
According to this question, a balanced chemical reaction between butane and oxygen is given as follows: 2C4H10 + 13O2 → 8CO2 + 10H2O.
The number of moles of butane is 2 while that of carbon dioxide is 8, therefore, the mole ratio of butane to carbon dioxide is 2:8 ~ 1:4.
Learn more about mole ratio at: https://brainly.com/question/12099869
Answer: A
Explanation: 2:8
Related Questions
How many particles of silver is 0.567 mol silver?
Answers
Thank you

how many electrons does Mg-3 have?
PLEASE HELP
Answers
Answer:
A magnesium ion mg-2 has A 24 protons and 22 electrons
How many moles of fluorine atoms are in a sample of fluorine that contains 7.45 x 10^24 fluorine atoms? (Report your answer to one place past the decimal point.
Answers
Based on the calculations, the number of moles of fluorine atoms present is equal to 12.4 moles.
How to calculate the moles of fluorine atoms?In order to determine the number of moles of fluorine atoms, we would calculate the number of atoms in 1 mole of an fluorine atoms in accordance with Avogadro's constant.
1 mole of fluorine atom = 6.02 × 10²³ molecules
X moles of fluorine atom = 7.45 × 10²⁴ molecules
Cross-multiplying, we have:
X = 7.45 × 10²⁴/6.02 × 10²³
X = 12.4 moles.
Read more on moles here: https://brainly.com/question/27952083
#SPJ1
An object has a mass of 5.4g and is 2.2cm long 1.7cm high and 0.8 cm wide what is the density will it float on water:
Answers
The density of an object is its mass divided by its volume. To find the volume of the object, we can use the formula for the volume of a rectangular prism:
V = l * w * h
Where:
V = Volume
l = length
w = width
h = height
In this case:
V = 2.2cm * 0.8cm * 1.7cm = 2.976 cm^3
To convert the mass from grams to grams per cubic centimeter, we need to convert the volume from cubic centimeters to cubic meters.
1cm^3 =10^-6 m^3. so 2.976cm^3 = 2.976*10^-6 m^3
So the density of the object is:
density = mass/volume = 5.4g / 2.976*10^-6 m^3 = 1817.5 kg/m^3
Now we can compare the density of the object to the density of water which is 1000 kg/m^3.
Since the density of the object is greater than the density of water, the object will not float on water.
true/false. the melting point of xeo2f2 (diagrams a,b, c) is greater than the melting point of xeo3f2 (diagram e.) identify the type(s) of intermolecular force(s) that the two substances have in common.
Answers
The given statement "the melting point of XeO₂F₂ (diagrams a, b, c) is greater than the melting point of XeO₃F₂" is true because both compounds, XeO₂F₂ and XeO₃F₂, have similar intermolecular forces.
London dispersion forces are present in all molecules, including nonpolar ones, and arise from temporary fluctuations in electron distribution around the molecules. These forces increase with the size of the molecule and the surface area available for contact. Since both compounds contain xenon, oxygen, and fluorine atoms, they have similar London dispersion forces.
Dipole-dipole interactions occur between molecules with permanent dipoles, such as polar molecules. In both XeO₂F₂ and XeO₃F₂, the highly electronegative fluorine and oxygen atoms create polar bonds with the xenon atom, resulting in molecular dipoles. The positively charged regions of one molecule are attracted to the negatively charged regions of a neighboring molecule, leading to dipole-dipole interactions.
The greater melting point of XeO₂F₂ compared to XeO₃F₂ can be attributed to the difference in the strength of these intermolecular forces. Since XeO₂F₂ has a more significant molecular mass and a larger surface area, its London dispersion forces are stronger.
Additionally, the molecular structure and the presence of the extra fluorine atom in XeO₂F₂ might contribute to stronger dipole-dipole interactions. These factors result in a higher melting point for XeO₂F₂ compared to XeO₃F₂.
Know more about melting point here:
https://brainly.com/question/29578567
#SPJ11
How do you find the ideal and actual MAs of the pulley systems?
Answers
Answer: The mass M = 9g, so G = 9g x 9.8 m/s² = 88.2gm/s², or 88.2 newtons. Insert the tension and gravitational force you just calculated into the original equation: -F = T + G = 18N + 88.2N = 106.2N. The force is negative because the object in the pulley system is accelerating upwards.
Explanation:
why is it more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume? byu
Answers
It is more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume because the amount of the material left in the trash will be less.
The extraction of certain ratio of the solute is able to the distribute among the phases during each extraction. The various extractions with the lesser amounts of the solvent are more efficient than the single extraction with the huge amount of solvent.
The extraction is about to maximize the outside field of the communication between the two solvents, we can easily get the more surface area in the contact with the fewer amounts.
To learn more about extraction here
https://brainly.com/question/14522836
#SPJ4
Place these elements in order of LOWEST electronegativity to HIGHEST.
= Barium (Ba)
= Cobalt (Co)
= Molebdenum (Mo)
= Nitrogen (N)
Answers
Answer:
Barium
Molebdenum
Cobalt
Nitrogen
Explanation:
science
Answer:
4= Barium (Ba) 2,6
2= Cobalt (Co) 1,91
3= Molebdenum (Mo) 1,6
1= Nitrogen (N) 3,04
Explanation:
How much energy is required to raise the temperature of 0.2 kg of aluminum
from 15°C to 18°C?
5382 J
B. 538 J
C. 600 J
D. 179 J
Answers
please im even confused with what to do ;-;

Answers
Answer:
Explanation:
The C option is the correct answer
Answer:
1) MEASURING CYLINDER as the name suggest it is cyllindrical also it is used in measure the volume of a liquid. It has a narrow cylindrical shape
2) BURRETE a graduated glass tube with a tap at one end, for delivering known volumes of a liquid, especially in titrations.
3) Pipette To transport a measured volume of liquid
SO THE ANSWER IS C, hope it helps
What is the molarity of .65 L of solution containing 63 grams of NaCl? (dont forget to convert grams to moles)
Answers
Answer:
1.66 M
Explanation:
Molarity = moles/L solution
We have the following data:
mass NaCl = m = 63 g
volume of solution = V = 0.65 L
Thus, we first convert the mass to moles with the molar mass of NaCl (MM):
MM(NaCl) = 23 g/mol Na + 35.4 g/mol Cl = 58.4 g/mol
moles NaCl = m/MM = 63 g/(58.4 g/mol)= 1.08 mol
Finally, we divide the moles into the volume of solution to calculate the molarity:
M = moles NaCl/V = 1.66 mol/L
solve the ivp d2ydt2 6dydt 34y=0,y(0)=0,y′(0)=−8 the laplace transform of the solutions is l{y}
Answers
the Laplace transform of the solution to the given initial value problem is Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i)
What is Laplace Transformation?
Laplace transform can be solved using the definition, properties, and techniques of Laplace transforms.
Laplace transform is a mathematical tool used to solve linear differential equations. The Laplace transform is defined as the integral of a function multiplied by an exponential function.
To solve the initial value problem (IVP) with the differential equation d²y/dt² + 6dy/dt + 34y = 0, and the initial conditions y(0) = 0 and y'(0) = -8, we can use the Laplace transform.
Taking the Laplace transform of the given differential equation, we have:
s²Y(s) - sy(0) - y'(0) + 6sY(s) - 6y(0) + 34Y(s) = 0
Applying the initial conditions, we substitute y(0) = 0 and y'(0) = -8:
s²Y(s) - 8s + 6sY(s) + 34Y(s) = 0
Rearranging the terms and factoring out Y(s), we get:
Y(s) (s² + 6s + 34) = 8s
Dividing both sides by (s² + 6s + 34), we find:
Y(s) = 8s / (s² + 6s + 34)
Now, we need to decompose the denominator into its quadratic factors:
s² + 6s + 34 = (s + 3 + 5i)(s + 3 - 5i)
Using partial fraction decomposition, we can express Y(s) as:
Y(s) = A / (s + 3 + 5i) + B / (s + 3 - 5i)
Multiplying through by (s + 3 + 5i)(s + 3 - 5i), we get:
8s = A(s + 3 - 5i) + B(s + 3 + 5i)
Expanding and equating the coefficients of s, we find:
8 = A + B
0 = 3B - 5A
Solving these equations simultaneously, we find A = -4/17 and B = 36/17.
Substituting these values back into the equation for Y(s), we get:
Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i)
Now, taking the inverse Laplace transform, we can find the solution y(t):
y(t) = L⁻¹{Y(s)}
The inverse Laplace transform of each term can be found using the table of Laplace transforms or by using software. The solution y(t) will involve a combination of exponential functions and trigonometric functions.
Therefore, the Laplace transform of the solution to the given initial value problem is Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i), and the inverse Laplace transform of Y(s) will give us the solution y(t) to the initial value problem.
To learn more about Laplace Transform from the given link
https://brainly.com/question/30402015
#SPJ4
Which element in the noble gas family has the largest atomic mass?
Answers
Answer:
Organessen
Explanation:
The largest element in the nobel gas family is Og, organessen. Element 188, It has an atomic mass of 294, far ahead of it's closest competitor, Radon (at 222). Organessen was synthesized in Russia in 2002 and only 6 atoms have been reported. One may question whether it really should be labelled a nobel gas, but it lies in the correct column. If giving such a high distinction to such a empheral element makes you queasy, vote for Radon in the next contest.
Ayúdenme por favor
Please help me
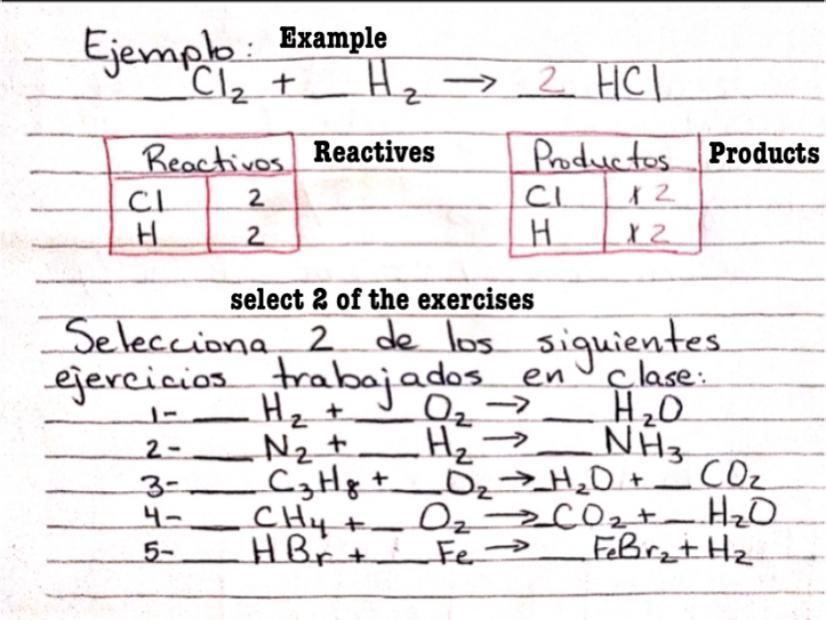
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
Which one of the following substances is an element?
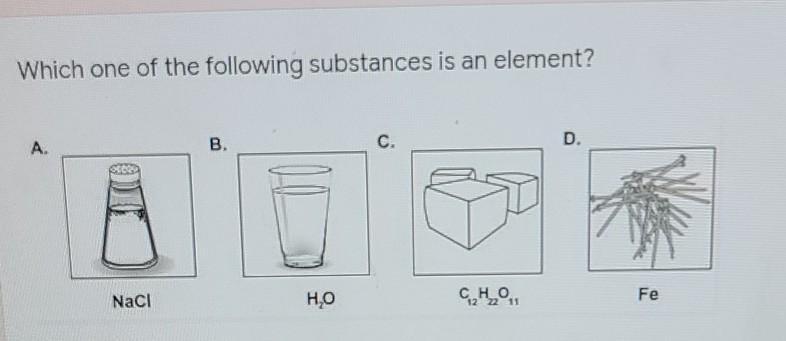
Answers
Answer: Fe
Explanation: Fe (Iron) is the only element since it involves only 1 atom. The other options are compounds, since there are more than 2 atoms bonded together.
lesson 5 researching economics
Answers
Economic research or research in economics helps to provide data that is relevant and crucial to making forecasts and decisions relating to the economic activity of a state or an organization.
How does Economic research help the economy?
Research and Development in the field of economics aid in ensuring that expenditure is wisely dissipated to ensure maximum national productivity.
Results from proper economic research can be used to create a framework for effective innovations and public policies.
Learn more about Economic research at:
https://brainly.com/question/10564789
(-7b + 8c) - (12a + 14) + (5a + 5b) =
Answers
Given problem;
(-7b + 8c) - (12a + 14) + (5a + 5b) = ?
To this problem, we open the brackets, collect like terms and factor them.
The order of operation, PEMDAS must be strictly adhered to;
P = Parentheses
E = Exponent
M = multiplication
D = Division
A = Addition
S = Subtraction
(-7b + 8c) - (12a + 14) + (5a + 5b);
Open the parentheses;
Note; + x + = +
+ x - = -
- x - = +
- x + = -
So,
= -7b + 8c -12a - 14 + 5a + 5b
Collect like terms;
= 5a -12a + 5b - 7b + 8c -14
= -7a - 2b + 8c -14
The solution is -7a - 2b + 8c -14
The room temperature BCC single-phase solid solution of carbon in iron is known as:
A. Austenite B. Ferrite C. Cementite D. Pearlite
B
Answers
The room temperature BCC single-phase solid solution of carbon in iron is known as B. Ferrite
Ferric is a type of iron that has been oxidized. It has a high oxidation state and is used in many industries. It is most commonly used as a catalyst in chemical reactions and as a pigment for paints, inks, and cosmetics. It is also used to create alloys and as a nutritional supplement. It is found in many foods, such as meats, eggs, nuts, whole grains, legumes, and leafy green vegetables.
The ferric BCC structure is a type of crystalline structure that is made up of iron atoms arranged in a body-centered cubic lattice. It is also known as the gamma-iron structure and is the most common type of iron crystal structure seen in nature.
The ferric BCC structure is composed of eight iron atoms arranged in a cube with one atom at the center and the other seven atoms located at the corners. The atoms are bound together through a combination of covalent and metallic bonds. The ferric BCC structure is the most thermodynamically stable form of iron at room temperature, making it the most common form seen in everyday life.
It has a high melting point and is highly resistant to corrosion. Its properties make it ideal for use in engineering applications such as automotive components, tools, and construction materials.
To know more about FCC, click below:
https://brainly.com/question/17111818
#SPJ4
What are the current and or future uses of genetically modified strawberries
Answers
In the future, genetically modified strawberries may become more widely available if they pass regulatory approvals and are deemed safe for consumption. They could potentially provide benefits such as reduced pesticide use, longer shelf life, and improved nutrition. However, there are also concerns about the environmental impact and potential health risks associated with genetically modified crops, which will need to be addressed before they can be widely adopted.
"
q2 Explain the following
diagenesis process and it is affects on permeability and
porosity.
b) cementation ( mineral cement) "
Answers
Cementation during the diagenesis process reduces the porosity and permeability of rocks by filling the pore spaces between grains with mineral cements, impacting fluid flow and rock strength.
Cementation is a process in which minerals precipitate and fill the spaces between sediment grains, binding them together. This process occurs as pore fluids, such as groundwater, carry dissolved minerals that can precipitate and form a solid cementing material.
During diagenesis, cementation can significantly impact the permeability and porosity of rocks. The precipitation of mineral cements fills the pore spaces between grains, reducing the overall porosity of the rock. This reduction in porosity limits the amount of fluid that can flow through the rock and decreases its permeability.
The type of mineral cement formed during cementation can also influence the strength and durability of the rock. Common mineral cements include calcite, silica, and iron oxides, which can impart different properties to the rock.
learn more about Porosity here:
https://brainly.com/question/29311544
#SPJ4
In the extraction of titanium from its ore, the final stage involves the reaction between titanium(IV) chloride, TiCl4,
and sodium.
TiCl4 + 4Na →→ Ti + 4NaCl
Calculate the maximum mass of titanium that can be obtained from 500 tonnes of titanium(IV) chloride in this
reaction.
(relative atomic mass: Ti = 48
relative formula mass of TiCl4 = 190)
(2)
Answers
The maximum mass of titanium that can be obtained from 500 tonnes of titanium(IV) chloride in this reaction is 24,000 tonnes.
The maximum mass of titanium500 tonnes x (1000 kg/tonne) x (190 g/mole TiCl4) = 95 x 10^6 g TiCl495 x 10^6 g TiCl4 x (48 g Ti/190 g TiCl4) = 24.7 x 10^6 g Ti.Therefore, the maximum mass of titanium that can be obtained from 500 tonnes of titanium(IV) chloride is 24.7 x 10^6 g.This can be calculated using the stoichiometry of the equation. First, calculate the number of moles of titanium(IV) chloride present in 500 tonnes.This is done by dividing the mass by the relative formula mass of the compound, giving 26,316 moles of TiCl4. The equation of the reaction shows that for every 1 mole of titanium chloride, 4 moles of sodium are required to produce 1 mole of titanium.Therefore, there are 104,264 moles of sodium required for the reaction. Since the mass of sodium is the same as its molar quantity, the mass of sodium required is 104,264 tonnes. Since the ratio of titanium produced to titanium chloride used is 1:1, the mass of titanium produced is 26,316 tonnes.This is equal to 24,000 tonnes when converted to kilograms. This process is known as stoichiometric calculations.To learn more about The maximum mass of titanium refer to:
https://brainly.com/question/30004075
#SPJ1
How many moles of NO2 form when
63.25 g N2O5 decompose?
2N2O5→ 4NO2 + 0₂
Answers
Answer:
1.1713 moles
Explanation:
RFM of N2O5= (14*2)+(16*4)=108
Moles of N2O5= Mass/RFM= 63.25/108= 0.5856 moles
Mole ratio of N2O5:NO2 = 2:4
Therefore moles of NO2= 4/2*0.5856= 1.1713 moles
The
mixture of C3H8 and C4H10 burned and get 3.74g CO2 and 1.98g H2O.
What is the ratio of moles of C3H8 and C4H10 in the original
mixture ?
Please help this ! Thanks
Answers
To determine the ratio of moles between C3H8 (propane) and C4H10 (butane) in the original mixture, we need to use the stoichiometry of the combustion reaction and the given amounts of CO2 and H2O produced.
First, let's calculate the moles of CO2 and H2O produced:
Molar mass of CO2 = 12.01 g/mol (C) + 2 * 16.00 g/mol (O) = 44.01 g/mol
Moles of CO2 = 3.74 g / 44.01 g/mol ≈ 0.085 mol
Molar mass of H2O = 2 * 1.01 g/mol (H) + 16.00 g/mol (O) = 18.02 g/mol
Moles of H2O = 1.98 g / 18.02 g/mol ≈ 0.11 mol
Next, we can set up the balanced equation for the combustion of propane (C3H8) and butane (C4H10):
C3H8 + 5O2 → 3CO2 + 4H2O (propane)
C4H10 + 6.5O2 → 4CO2 + 5H2O (butane)
From the balanced equations, we can see that for every 3 moles of CO2 produced, there are 1 mole of C3H8 and for every 4 moles of CO2 produced, there are 1 mole of C4H10.
Now, we can calculate the moles of C3H8 and C4H10 based on the moles of CO2 produced:
Moles of C3H8 = 0.085 mol * (1 mol C3H8 / 3 mol CO2) ≈ 0.0283 mol
Moles of C4H10 = 0.085 mol * (1 mol C4H10 / 4 mol CO2) ≈ 0.0213 mol
Therefore, the ratio of moles between C3H8 and C4H10 in the original mixture is approximately 0.0283 mol : 0.0213 mol, which simplifies to 1.33 : 1 (or approximately 4 : 3).
To know more about stoichiometry, visit:
https://brainly.com/question/24384921
#SPJ11
I stg these bots with links keep answering my questions so can anyone help me with this problem?
How many grams of chlorine gas are needed to react with 2.50 liters of a 3.34 molar potassium bromide solution?
Cl2 + 2KBr → 2KCl + Br2
Answers
According to the website that I use to figure out things about science or chemistry and that stuff... The answer is 3.5 L KBr x 1.7 mol KBr solute / 1 L KBr solution x 1 mol Cl2 / 2 mol KBr x 70.9 g Cl2 / 1 mol Cl2 = 211 g Cl2.
table sugar (sucrose) can be dehydrated to make activated carbon. assuming the collection of the activated carbon is in 100% yield, what mass of sugar is needed to make 733.2 g of carbon.
Answers
To make 733.2 g of carbon we need 1710g of sucrose.
For the dehydration of 1 mol of sucrose we get 144 gram of sucrose thus to get 733.2 g of carbon we need \(\frac{733.2}{144}\) mol = 5 mol of sucrose, and 1 mol of sucrose = 342 g of sucrose so 5 mol means 5*342= 1710 mol.
Dehydration: Simply meaning is the removal of water molecule from the compound. \(C_{12} H_{22} O_{11}\)→12C+11\(H_{2} O\)
Read more about dehydration:
brainly.com/question/22733625
#SPJ4
Suppose you add 2.4x10^-3 moles of HNO3 to enough water to make 792 mL of solution What is the pH of the solution?
URGENT
Answers
2.4 x 10^-3 moles HNO3 / 0.792 L solution = 0.00303 M HNO3
Since HNO3 is a strong acid, it will dissociate completely in solution to form H+ and NO3- ions. Therefore, the concentration of H+ ions in the solution is also 0.00303 M.
To find the pH of the solution, we can use the formula:
pH = -log[H+]
pH = -log(0.00303)
pH = 2.52
Therefore, the pH of the solution is 2.52.
The partial pressures of ch4, n2, and o2 in a sample of gas were found to be 143 mmhg, 469 mmhg, and 251 mmhg, respectively. Calculate the mole fraction of oxygen.
Answers
The mole fraction of oxygen gas is 0.290
First we will calculate the total pressure by adding the partial pressure of all gases as follows:
Partial pressure of CH₄= 143 mmHg
Partial pressure of N₂= 469mmHg
Partial pressure of O= 251mmHg
Total pressure = partial pressure of CH₄+ partial pressure of N₂+ partial pressure of O₂
Total pressure= 143+ 469+ 251
=863mmHg
Finally we will calculate the mole fraction of O₂ with the help of formula written below:
Mole fraction=\(\frac{partial pressure }{total pressure}\)
Mole fraction of O₂=\(\frac{251}{863}\)
Mole fraction of O₂= 0.290
Therefor answer will be 0.290
To look more about Mole fraction click here
brainly.com/question/8076655
#SPJ4
help pleasee! will
give brainliest +80 pts

Answers
Answer: type the compound into your search bar then it should tell you what it is for 3. you should look up "what is the formula for -----" same thing for #4
Explanation:
Answer:
2.a. germanium tetrahydride
2.b. dinitrogen tetrabromide
2.c. diphosphorus pentasulfide
2.d. selenium dioxide
2.e. nitrogen trihydride
2.f. silicon dioxide
3.a. PO3
3.b. SiCl4
3.c. N2O5
3.e. N2O4
3.f. CO
4.a CO2
4.b. SF6
4.c. N2Cl4
4.d. CI4
4.e. PF5
4.f. P2O5
****All numbers are subscripts, please do not write them as is, but to the bottom right of them like shown in the options from question 2.
Explanation:
To name covalent compounds (NM+NM), we use prefixes.
To name covalent compounds goes as follows:
First, name the first element in the formula the normal name it has (ex. Nitrogen, Oxygen). If the first element is present more than once shown by a subscript, use a prefix that will indicate how many there are present (ex. mono, di, tri).
Next, name the second element in the compound using prefixes aswell if present more than once. These elements though, will end with -ide instead of their original name (ex. monoxide, dibromide, trichloride).
Which of the following elements would you expect to have the most stable nuclides? Element number: a. 47. b. 48. c. 50. d. 51. e. 52.
Answers
Among the given elements, tin (Sn) with element number c is expected to have the most stable nuclides due to its relatively low atomic number and a neutron-to-proton (N/Z) ratio close to 1. Here option C is the correct answer.
To determine which element among a, b, c, d, and e would have the most stable nuclides, we need to consider the concept of nuclear stability. The stability of a nucleus is influenced by the balance between the strong nuclear force, which holds the nucleus together, and the electrostatic repulsion between protons within the nucleus.
One way to evaluate nuclear stability is to examine the neutron-to-proton (N/Z) ratio. In general, for lighter elements, a stable nucleus tends to have an N/Z ratio close to 1, while for heavier elements, the ratio tends to increase. Elements with a larger number of protons (Z) require more neutrons (N) to stabilize the nucleus against repulsive forces.
Now, let's analyze the given elements:
a. Element 47 is silver (Ag).
b. Element 48 is cadmium (Cd).
c. Element 50 is tin (Sn).
d. Element 51 is antimony (Sb).
e. Element 52 is tellurium (Te).
Among these elements, tin (Sn) with element number c has the most stable nuclides. Tin has a relatively low atomic number, and its N/Z ratio is close to 1, making it more stable compared to the other elements listed. As we move towards heavier elements, the N/Z ratio increases, indicating a less stable nucleus.
To learn more about nuclides
https://brainly.com/question/32085983
#SPJ4
the objective portion of a soap note contains the
Answers
The objective portion of a soap note contains the measurable and observable information regarding the patient's physical examination and vital signs.
This includes information such as blood pressure, heart rate, respiratory rate, temperature, height, weight, and any physical findings from the exam. It is a crucial component of the soap note as it provides important details about the patient's current health status and helps healthcare providers make informed decisions regarding their care plan.
SOAP is an acronym for the 4 sections, or headings, that each progress note contains:
Subjective: Where a client’s subjective experiences, feelings, or perspectives are recorded. This might include subjective information from a patient’s guardian or someone else involved in their care.
Objective: For a more complete overview of a client’s health or mental status, Objective information must also be recorded. This section records substantive data, such as facts and details from the therapy session.
Assessment: Practitioners use their clinical reasoning to record information here about a patient’s diagnosis or health status. A detailed Assessment section should integrate “subjective” and “objective” data in a professional interpretation of all the evidence thus far, and
Plan: Where future actions are outlined. This section relates to a patient’s treatment plan and any amendments that might be made to it.
To learn more about soap note https://brainly.com/question/9306959
#SPJ11