Suppose an Oklahoman comes to Texas to go turkey hunting. He takes up camp 150 meters from the base of a cliff. When he sees a turkey he raises his shotgun, shoots, but misses (being a poor shot). He notices that he hears the echo of his gun off the cliff. How much time (in seconds) passes after the shot before the hunter hears the echo?
Answers
Answer:
0.44s
Explanation:
Given parameters:
Distance from the turkey = 150m
Unknown:
Time taken to hear the sound = ?
Solution:
The speed of sound is the distance traveled per unit of time for a sound wave.
It is given as a constant which is 343m/s
As with this parameter:
Speed = \(\frac{distance}{time}\)
Time = \(\frac{distance}{speed}\) = \(\frac{150}{343}\) = 0.44s
Related Questions
When revising an essay, how can a writer best connect ideas more clearly?
Answers
Answer: Using transitions to create a logical relationship among the claim, reasons, and evidence.
Calculate the number of atoms in a 3.68×103 g sample of aluminum.
Answers
Answer:
84.23×10^23
Explanation:
no.of atoms =given wt / gram Mw×avagdro num
Help me answer this please
Are the electrons in the following redox reactions transferred completely from the atoms of one element to the atoms of another or are they only partially transferred?
a. Ca(s) + Cl2(g) → CaCl2(s)
b. 4Cu(s) + O2(g) → 2Cu2O(s)
Answers
The electrons, in redox reaction get partially transferred by one to another element.
What is redox reaction?A redox reaction occurs when the oxidation states of the substrate change. The loss of electrons or maybe an increase in the oxidation state of a chemical and its atoms is referred to as oxidation. The gain of electrons or a reduction in the oxidation state of either a chemical or its atoms is referred to as reduction. The oxidation as well as reduction process can be seen in same reaction which is introduced as redox reaction.
What is electrons?
Electron can be considered as sub atomic particle which carry negative charge on it.
The given reactions are:
a. Ca(s) + Cl2(g) → CaCl2(s)
b. 4Cu(s) + O2(g) → 2Cu2O(s)
After transferring two electron in both reaction Ca and Cu it will form CaCl2 and 2Cu2O.
Therefore, after transferring partially electrons one elements get converted into another kind of molecule
To know more about redox reaction click here.
https://brainly.com/question/13293425.
#SPJ2
How many milliliters of a 0.1800 M solution of KOH will be required to titrate 44.00 mL of a 0.0683 M solution of H3PO4?
Answers
What compound has 4 hydrogen atoms and one carbon
Answers
Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4start subscript, 4, end subscript), carbon forms covalent bonds with four hydrogen atoms
__________________________________________________________
Give an example of how matter flows through a system.
Answers
Answer:
Carbon and nitrogen are examples of nutrients. Decomposers release nutrients when they break down dead organisms. The nutrients are taken up by plants through their roots. The nutrients pass to primary consumers when they eat the plants.
Explanation:
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
One of the commercial uses of sulfuric acid is in the production of calcium sulfate and phosphoric acid. If 23.7 g of Ca₃(PO₄)₂ reacts with an excess of H₂SO₄, what is the percent yield if 13.4 g of H₃PO₄ are formed in the following UNBALANCED chemical equation
Answers
The percentage yield would be 90.42%
First, the balanced equation of the reaction is as follows:
Ca₃(PO₄)₂ (s) + 3H₂SO₄ (aq) → 2H₃PO₄ (aq) + 3CaSO₄ (aq)
From the equation, the mole ratio of Ca₃(PO₄)₂ and H₃PO₄ is 1:2
Recall that: mole= mass/molar mass
Mole of Ca₃(PO₄)₂ = 23.7/310.174
= 0.076 mole
Mole ratio of Ca₃(PO₄)₂ and H₃PO₄ = 1:2
Mole of H₃PO₄ = 0.076 x 2
= 0.153 mole
Theoretical yield (in g) of H₃PO₄ = mole x molar mass
= 0.153 x 97.994
= 14.82 g
Percentage yield of H₃PO₄ = actual yield/theoretical yield x 100
= 13.4/14.82 x 100
= 90.42%
More on percentage yield of reactions can be found here: brainly.com/question/20758645
Sample Response: Matter is any substance that has
mass and takes up space. It can be identified using
physical properties such as density. Matter can change
states, which can be measured as boiling and melting
points. It can also be identified based on chemical
properties such as reactivity, flammability, and
composition.
What did you include in your response? Check all that
apply.
Matter is any substance that has mass and takes up
space.
Matter can be identified using physical properties
such as density and the boiling and melting points.
Matter can be identified based on chemical
properties such as reactivity, flammability, and
composition.
Done
Answers
The responses that can be included are : matter is any substance that has mass and takes up space, matter can be identified using physical properties, such as density and the boiling and melting points, matter can be identified based on chemical properties such as reactivity, flammability, and composition.
Matter is any substance that has mass and takes up space. This is a fundamental characteristic of matter, distinguishing it from non-material entities. Matter can be identified using physical properties such as density. Density is the measure of how much mass is contained in a given volume of a substance. Additionally, the boiling and melting points of matter can also be used to identify it. These points represent the temperatures at which matter changes from one state to another (e.g., from a solid to a liquid or from a liquid to a gas). Matter can also be identified based on its chemical properties. Chemical properties include reactivity, which refers to how a substance interacts with other substances; flammability, which indicates the ability to burn or ignite; and composition, which refers to the types and proportions of elements or compounds present in a substance. These characteristics and properties help scientists categorize and understand different forms of matter, enabling them to study and manipulate it for various purposes, such as in chemistry, physics, and engineering.
For such more questions on flammability
https://brainly.com/question/2583069
#SPJ11
The half-life of radon-222 is 4 days. How much of a 100g sample would be left after 8 days?
Answers
The half-life of radon-222 is 4 days, which means that after each 4-day period, the amount of radon-222 in a sample is halved.
After 8 days, only 25 g of the 100 g sample of radon-222 would be remaining. After 8 days, there have been two half-life periods. Therefore, we can find the amount of radon-222 remaining after 8 days using the following formula:
Amount remaining = (Initial amount) x (1/2)^(number of half-life periods)
Initial amount = 100 g
Number of half-life periods = 8 days / 4 days per half-life = 2 half-life periods
Substituting these values into the formula gives:
Amount remaining = 100 g x (1/2)² = 100 g x (1/4) = 25 g
Therefore, after 8 days, only 25 g of the 100 g sample of radon-222 would be remaining.
To know more about half life please refer:
https://brainly.com/question/24710827
#SPJ1
Using the average concentration of vitamin C determined in the experiment, how many fluid ounces of fruit juice should you drink, in order to consume 75 mg of vitamin C, which
is the Dietary Guideline of Vitamin C per day for women? (33.8 fluid ounce = 1 liter).
Average concentration of vitamin C = 1.602 mL
Answers
You should drink 1.6 × 10² fl oz of fruit juice with a vitamin C concentration of 1.602 mg/100mL in order to consume 75 mg of vitamin C.
The average concentration of vitamin C in the given fruit juice is 1.602 mg/100mL, that is, there are 1.602 mg of vitamin C per 100 mL of juice. The volume of juice that contains 75 mg of vitamin C is:
\(75mg\ vitamin\ C \ times \frac{100mL\ Juice}{1.602mg\ vitamin\ C} = 4.7 \times 10^{3} mL\ Juice\)
Then, we will convert 4.7 × 10³ mL to fluid ounce using the following conversion factors:
1 L = 1000 mL1 L = 33.8 fl oz\(4.7 \times 10^{3} mL \times \frac{1L}{1000mL} \times \frac{33.8 fl\ oz}{1L} = 1.6 \times 10^{2} fl\ oz\)
You should drink 1.6 × 10² fl oz of fruit juice with a vitamin C concentration of 1.602 mg/100mL in order to consume 75 mg of vitamin C.
You can learn more about unit conversion here: https://brainly.com/question/19420601
Determine the number of moles in 258.45 grams of iron (III) chloride (FeCl3). Show and explain all
your work for full credit. Make sure you show all units.
Answers
Answer:
\(\boxed {\boxed {\sf 1.5935 \ mol \ FeCl_}}\)
Explanation:
To convert from grams to moles, we must use the molar mass. This can be found on the Periodic Table. First, find the molar mass of iron and chlorine.
Fe: 55.84 g/mol Cl: 35.45 g/molCheck the formula. There is a subscript of 3 after Cl, so there are 3 atoms of chlorine in 1 molecule. Multiply iron's molar mass by 3, then add iron's molar mass.
FeCl₃: 55.84 + 3(35.45) = 55.84+106.35=162.19 g/molUse this number as a ratio.
\(\frac {162.19 \ g\ FeCl_3}{1 \ mol \ FeCl_3}\)
Multiply by the given number of grams.
\(258.45 \ g \ FeCl_3 *\frac {162.19 \ g\ FeCl_3}{1 \ mol \ FeCl_3}\)
Flip the ratio so the grams of iron (III) chloride cancel.
\(258.45 \ g \ FeCl_3 *\frac {1 \ mol \ FeCl_3}{162.19 \ g\ FeCl_3}\)
\(258.45 *\frac {1 \ mol \ FeCl_3}{162.19}\)
\(\frac {258.45 \ mol \ FeCl_3}{162.19}\)
\(1.59350144892 \ mol \ FeCl_3\)
The original measurement of grams has 5 significant figures, so our answer must have the same. For the number we calculated, that is the ten thousandth place.
\(1.5935 \ mol \ FeCl_3\)
258.45 grams is approximately 1.5935 moles of iron (III) chloride.
How many grams of carbon dioxide are produced when 191 g of methane (CH4) are burned
completely as shown in the reaction below:
CH4 + 202 + CO2 + 2H2O
Answers
Answer:
524g CO2
Explanation:
Just took the quiz
How much energy must be removed from 13.9 grams of gaseous oxygen in order to decrease the temperature from 38.8°C to 23.8°C? The specific heat of oxygen is 0.219 cal/g °C. in cal
Answers
So,
To solve this question, we could use the equation:
What we're going to do now is to replace the values that we know:
The negative sign of the result indicates that this heat will be lost.
Therefore, the amount of energy that must be removed, equals 45.6615 cal.


explain how to balance the chemical equation and classify its reaction type.
___C5H5 +___ Fe ⟶ ___Fe(C5H5)2
Answers
Answer: The balanced chemical equation is given below.
Explanation:
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
The balanced chemical equation follows:The balanced chemical equation follows:
On reactant side:
Number of carbon atoms = 10
Number of hydrogen atoms = 10
Number of iron atoms = 1
On product side:
Number of carbon atoms = 10
Number of hydrogen atoms = 10
Number of iron atoms = 1
So, the balanced chemical equation is given above.
Answer:
#1. Balanced equation: 2C₅H₅ + Fe → Fe(C₅H₅)₂
#2. Type of reaction: Synthesis reaction
Explanation:
Balanced equations are equations that obey the law of conservation of mass.
When an equation is balanced the number of atoms of each element is equal on both side of the equation.
Equations are balanced by putting appropriate coefficients on the reactants and products.
In our case, we are going to put coefficients 2, 1 and 1.
Thus, the balanced equation will be;
2C₅H₅ + Fe → Fe(C₅H₅)₂
This type of a reaction is known as synthesis reaction, in which two or more reactants or compounds combine to form a single compound or product.
Question 7 of 10
When naming a molecule, how do you indicate that the carbons on either side
of the double bond are pointing in opposite directions?
OA. Use the prefix cis-.
OB. Use the prefix methyl-.
OC. Use the number 2 as a prefix.
OD. Use the prefix trans-.

Answers
Answer:
use the prefix trans-
Explanation:
trans = opposite orientation across double bond its this one for apex
cis = same orientation across double bond.
Hopefully this helps! :)
Consider the oxidation of sodium metal to sodium oxide described by the balanced equation:
4 Na + O2 → 2 Na2O. What is the theoretical yield of Na2O in grams from 9.0 mol of O2?
show steps
Answers
Answer:
1116 g.
Explanation:
The balanced equation for the reaction is given below:
4Na + O₂ —> 2Na₂O
From the balanced equation above,
1 mole of O₂ reacted to produce 2 moles of Na₂O.
Next, we shall determine the theoretical yield of Na₂O. This can be obtained as follow:
From the balanced equation above,
1 mole of O₂ reacted to produce 2 moles of Na₂O.
Therefore, 9 moles of O₂ will react to produce = 9 × 2 = 18 moles of Na₂O.
Finally, we shall determine the mass in 18 moles of Na₂O. This can be obtained as follow:
Mole of Na₂O = 18 moles
Molar mass of Na₂O = (23×2) + 16
= 46 + 16
= 62 g/mol
Mass of Na₂O =?
Mass = mole × molar mass
Mass of Na₂O = 18 × 62
Mass of Na₂O = 1116 g
Thus, the theoretical yield of Na₂O is 1116 g.
Melissa found that when she added 965 J of energy to a 0.25-kg sample of copper, the temperature of the copper increased by 10 K. What is the specific heat of copper? Show your work.
Answers
Answer:
c = 14.668 J/kg°C
Explanation:
Given that,
Heat added, Q = 965 J
Mass of the sample, m = 0.25 kg
The change in temperature, \(\Delta T=10\ K=-263.15^{\circ}C\)
We need to find the specific heat of the copper. The heat required to raise the temperature is given by :
\(Q=mc\Delta T\\\\c=\dfrac{Q}{m\Delta T}\\\\c=\dfrac{965\ J}{0.25\ kg\times -263.15^{\circ} C}\\\\c=14.668\ J/kg^{\circ} C\)
So, the specific heat of the copper is 14.668 J/kg°C.
Why is scientific notation used?
to round numbers to the nearest whole number
to promote reproducibility of data
to increase the validity of data
to express very large or very small numbers
Answers
The correct option is (d) To express very large or very small number.
What is the Scientific Notation?
Scientific notation is a way to present numbers that are too large or too small to be easily written in decimal form. The three components of scientific notation are coefficient, base and exponent. The proper format to write a scientific notation is a x 10^b, where a is a number or decimal number and b is the power of 10 to make scientific notation equivalent to original number. When a number between 1 and 10 is multiplied by a power of 10 then the number is expressed in scientific notation. For example, 10000000 can be written as 10⁷, which is the scientific notation and the exponent is positive here. Similarly, for the negative exponent 0.000001 can be can be represented as 10-⁷. Hence, the scientific notation is used to express very large or very small numbers.
Learn more about Scientific Notation here: https://brainly.com/question/15361382
#SPJ1
An enzyme can catalyze a reaction with either of two substrates, S1 or S2. The Km for S1 was found to be 2.0 mM, and the Km, for S2 was found to be 20 mM. A student determined that the Vmax was the same for the two substrates. Unfortunately, he lost the page of his notebook and needed to know the value of Vmax. He carried out two reactions: one with 0.1 mM S1, the other with 0.1 mM S2. Unfortunately, he forgot to label which reaction tube contained which substrate. Determine the value of Vmax from the results he obtained:
Answers
Answer:
101
Explanation:
Provided that
\(S_1 = S_2 = same\ V_{max}\)
And,
\(S_1\ k_M = 2.0mM\\S_2\ k_M = 20mM\)
Now we expect the same
{S} (0.1mM)
This determines that \(S_1\) generates a higher rate of product formation as compared to the \(S_2\)
So we can easily calculate the \(V_{max}\) for either of \(S_1\) or \(S_2\) as we know that Tube 1 is \(S_2\) and tube 2 is \(S_1\)
As we know that
\(V_0 = V_{max}\ {S} / (K_M + {S})\)
As the rates do not include any kind of units so we do not consider the units for \(V_{max}\)
Now the calculation is
\(0.5 = V_{max} (0.1\ mM) / (20\ mM + 0.1\ mM)\)
\(V_{max} = 0.5 (20.1\ mM) / 0.1\ mM\)
= 100.5
≈ 101
Calculate the energy needed to heat 10.5 g ice at -20.0 °C to liquid water at 75.0 °C. The heat of vaporization of water = 2257 J/g, the heat of fusion of water = 334 J/g, the specific heat capacity of water = 4.18 J/g·°C, and the specific heat capacity of ice = 2.06 J/g·°C.
Answers
Answer:
= 7234.5Joules
Explanation:
Q = (mcΔT)ice + (mΔH)melting +(mcΔT)water
= (10.5g)(2.06J/g°C)[0°C-(-20°C)+(10.5g)(2257J/g)+(10.5g)(4.184J/g°C(75°C - 0°C)
= [(10.5)(2.06)(20) + (10.5)(2257) + (10.5)(4.184)(75)]J
= 432.6J + 3507J + 3294.9J
= 7234.5Joules
how many bond can boron make without hybridization
Answers
Without hybridization, boron can form 4 bonds
What is hybridization?Hybridisation is phenomenon of combining two atomic orbitals to give a new degenerate hybrid orbital which have same energy levels. Hybridization increases the stability of bond formation than unhybridized orbitals. We can predict the shape of molecules by its hybridization.
With hybridization , boron can form 3 bonds.
Hence, without hybridization, Boron donates the lone pair of electrons to form the fourth bond. In addition to that Boron is a second period element hence, which makes it small in size and d-orbitals are unavailable as well. Hence, Boron can only form 4 bonds with hybridization.
learn more about hybridization from
https://brainly.com/question/22765530
#SPJ1
Why cant chemical changes be reversed
Answers
Answer:
Throughout the explanation section, the reason behind the given statement is described.
Explanation:
The chemicals thus produced were indeed opposite or separate from the reaction mixture, this same reaction wouldn’t change when it's more balanced than some of the reactants.Another reason is that the development of advanced organisms, as well as chemical alterations, is irreversible throughout nature cant undo the chemical modifications.If 50 joules of energy is added to sample of water, the temperature will?
Answers
Explanation:
The temperature change of a substance when it absorbs or loses energy can be calculated using the specific heat capacity of the substance. The specific heat capacity of water is approximately 4.18 J/(g°C), which means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
To calculate the temperature change of the water sample when 50 joules of energy is added, we need to use the following equation:
q = m * c * ΔT
where q is the amount of energy absorbed by the water, m is the mass of the water sample, c is the specific heat capacity of water, and ΔT is the resulting temperature change.
Rearranging the equation to solve for ΔT, we get:
ΔT = q / (m * c)
Plugging in the values, we get:
ΔT = 50 J / (m * 4.18 J/(g°C))
We need to know the mass of the water sample to calculate the temperature change. Let's assume a mass of 10 grams:
ΔT = 50 J / (10 g * 4.18 J/(g°C))
ΔT = 1.2°C
Therefore, if 50 joules of energy is added to a 10-gram sample of water, the resulting temperature change will be approximately 1.2 degrees Celsius.
Compare the average motion of the particles in the 3 containers of water
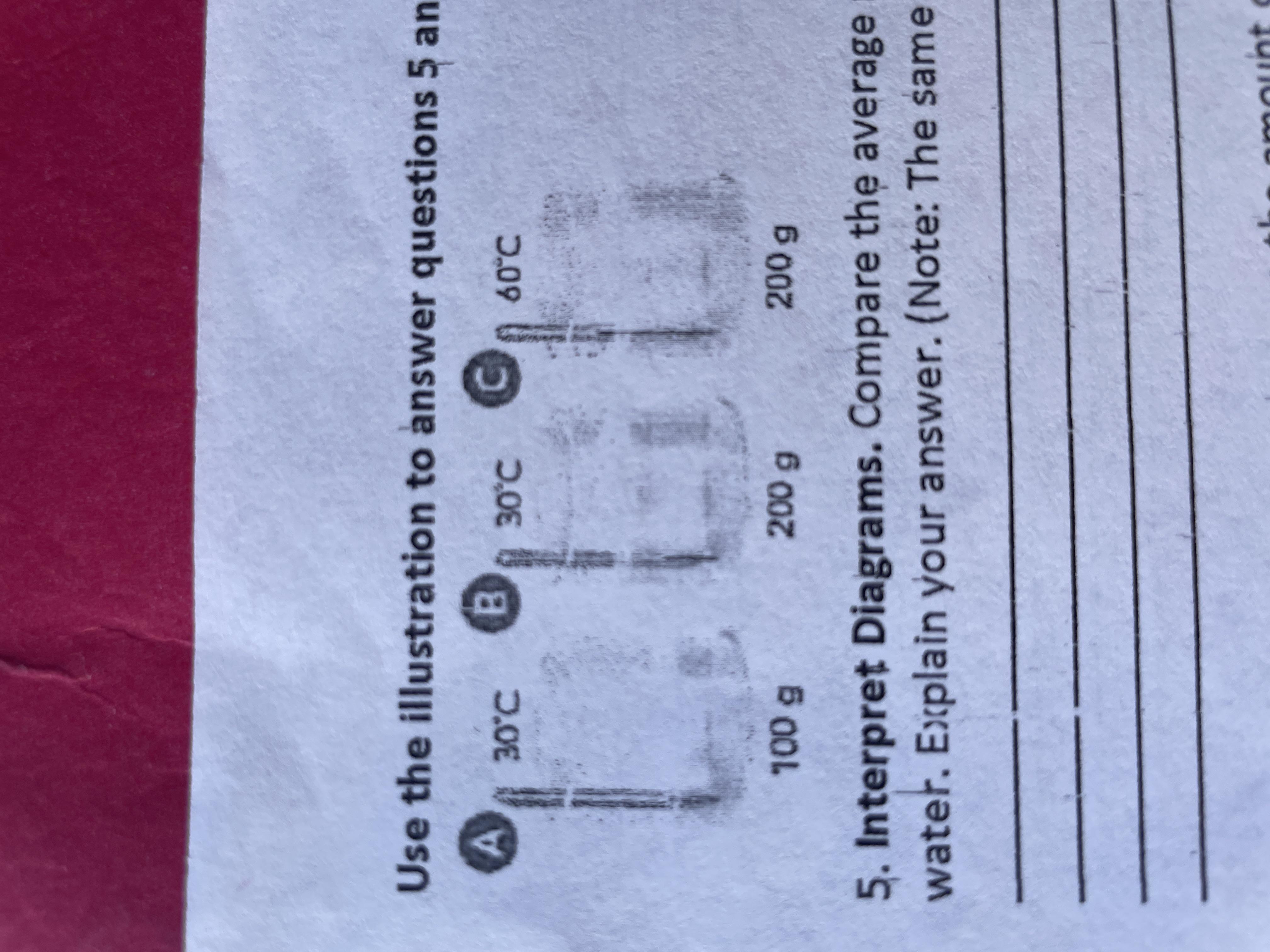
Answers
Answer:
c>b=a
Explanation:
It is important to note that mass does not affect the average motion/energy per molecule, but temperature does. the higher the temperature the faster the particles are. A has the same temperature as B, so they have the same amount of motion. C is warmer than A and B, so the average motion of the particles in beaker C is the largest
help please! predict whether each substance will dissolve in water.

Answers
The compound n - hexane as shown does not dissolve in water.
Why does n - hexane not dissolve in water?
Due to a large difference in polarity between the two compounds, n-Hexane does not dissolve in water.
Water is a polar molecule, which means that one end of it (the hydrogen side) has a slight positive charge and the other end (the oxygen side) has a slight negative charge. The unequal distribution of electrons within the water molecule, which produces a dipole moment, is the cause of this polarity.
N-Hexane, on the other hand, is a nonpolar molecule. The electrons are uniformly distributed throughout the molecule, which is made up of carbon and hydrogen atoms bound together in a straight chain. N-Hexane lacks a substantial dipole moment as a result.
Learn more abaout hexane:https://brainly.com/question/30364285
#SPJ1
How many moles are in 6.02x10^23 molecules of Br2
Answers
Answer:
1 mole = 6.02 x 1023 atoms
Explanation:
When the following solutions are mixed together, what precipitate (if any) will form?
a. Hg(NO,)(ag) + CuSO (ag)
b. Ni(NO,)(ag) + CaCh(ag)
c. K;COg(ag) + Mgl.(ag)
d. Na;CrO.(ag) + AlBs(ag)
Answers
The precipitates that will be formed respectively from the reactions would be:
\(HgSO_4\)No precipitate \(MgCO_3\) \(Al_2 (CrO_4)_3\)What are precipitation reactions?Precipitation reactions are reactions during which two aqueous salt solutions combine to produce one aqueous and one insoluble salt.
Following this definition, the precipitates that will be formed from each of the reactions can be deduced as follows:
\(Hg_2(NO_3)(aq) + CuSO_4 (aq) --- > HgSO_4 (s) + CuNO_3 (aq)\). The precipitate here is \(HgSO_4\)\(Ni(NO_3)_2(aq) + CaCl_2(aq) --- > NiCl_2 (aq) + Ca (NO_3)_2 (aq)\). No precipitate is formed here.\(K_2CO_3(aq) + Mgl_2(aq)--- > KI (aq) + MgCO_3 (s)\). The precipitate formed here is \(MgCO_3\)\(Na_2CrO_4(aq) + AlBr_3(aq) --- > NaBr (aq) + Al_2 (CrO_4)_3 (s)\). The precipitate formed here is \(Al_2 (CrO_4)_3\)The production of precipitates follows solubility rules. Some of the rules are:
All sodium salts are solubleAll chloride salts are solubleAll iodide salts are solubleAll nitrate salts are solubleMore on the formation of precipitates can be found here: https://brainly.com/question/17687281
#SPJ1
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
a forest is a habitat for many living things. what happens to these different living things
Answers
Forest is a habitat for many plants and animals because it provides a suitable environment for them.
Forest is a habitat for many plant life and animals as it offers a appropriate environment for them. As an instance, neem, Sheesham, Palash, tiger, porcupine, elephants, jackal and so forth. The ground beneath the tree of the forest is the forest floor.
It causes habitat destruction, accelerated chance of predation, reduced food availability, and lots greater. As a result, some animals lose their homes, others lose meals assets – and ultimately, many lose their lives. In reality, deforestation is one of the essential causes of extinction.
Weather trade also alters the life cycles of flora and animals. as an instance, as temperatures get warmer, many plant life are starting to develop and bloom earlier inside the spring and live to tell the tale longer into the fall. A few animals are waking from hibernation sooner or migrating at different instances, too.
Learn more about habitat here:- https://brainly.com/question/23780904
#SPJ9