Some chemical reactants are listed in the table below. Complete the table by filling in the oxidation state of the highlighted atom. species oxidation state of highlighted atom OH (aq) __
NH4 (aq) __
I (aq) __
Br2(g) __
Answers
The oxidation state of the highlighted atoms in the chemical species is as follows:
O in OH⁻ is -2N in NH₄ (aq) is -3I in I⁻ (aq) is -1B in Br₂ is 0What are the oxidation states of the atoms in the chemical reactants?An atom's oxidation number or oxidation state in a chemical species reveals how many electrons it has lost or gained in a compound or ion.
In OH⁻ (aq), the highlighted atom is oxygen (O), and its oxidation state is -2.
In NH₄ (aq), the highlighted atom is nitrogen (N), and its oxidation state is -3.
In I⁻ (aq), the highlighted atom is iodine (I), and its oxidation state is -1.
In Br₂(g), the highlighted atom is bromine (Br), and since it is in its elemental form, its oxidation state is 0.
Learn more about oxidation state at: brainly.com/question/25551544
#SPJ4
Related Questions
Using the following equation for the combustion of octane, calculate the heat of reaction for 400.0 g of octane. The molar mass of octane is 114.33 g/mole.2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
Answers
The heat of reaction for 400.0 g of octane is -19274 kJ.
Let's recalculate the heat of the reaction for 400.0 g of octane.
Calculate the number of moles of octane:
moles of octane = mass of octane / molar mass of octane
moles of octane = 400.0 g / 114.33 g/mol
Determine the heat of reaction per mole of octane:
ΔH°rxn per mole of octane = -11018 kJ / 2 moles
Calculate the heat of the reaction for 400.0 g of octane:
heat of reaction = ΔH°rxn per mole of octane × moles of octane
Now, let's substitute the values and calculate the heat of reaction:
moles of octane = 400.0 g / 114.33 g/mol = 3.497 mol
ΔH°rxn per mole of octane = -11018 kJ / 2 moles = -5509 kJ/mol
heat of reaction = -5509 kJ/mol × 3.497 mol ≈ -19274 kJ
Therefore, the heat of the reaction for 400.0 g of octane is approximately -19274 kJ.
know more about heat of reaction here:
brainly.com/question/30846564
#SPJ11
draw two linkage isomers of [mn(nh3)5(no2)]2+.
Answers
Linkage isomers are a type of coordination isomerism that occurs when ligands can coordinate to a central metal ion through different atoms. In the case of [Mn(NH3)5(NO2)]2+, two possible linkage isomers can be formed.
In one linkage isomer, the nitrite ion (NO2-) coordinates to the central manganese ion (Mn) through the nitrogen atom, forming a coordinated nitrito ligand. The ammonia molecules (NH3) then coordinate to the manganese ion.
In the other linkage isomer, the nitrite ion coordinates to the manganese ion through the oxygen atom, forming a coordinated nitro ligand. Again, the ammonia molecules coordinate to the manganese ion.
The difference between the two isomers lies in the coordination atom of the nitrite ligand, either nitrogen or oxygen. The arrangement of the ammonia ligands around the central manganese ion remains the same in both isomers.
1. Nitrito-N isomer: In this isomer, one of the nitrogen atoms of the nitrite ligand (NO2-) is coordinated to the manganese (Mn) atom. The remaining oxygen atom of the nitrite ligand remains uncoordinated. The five ammonia (NH3) ligands are coordinated to the manganese atom.
Structural formula:
NH3
|
Mn -- NH3
|
NH3
|
NH3
|
NO2
2. Nitrito-O isomer: In this isomer, the oxygen atom of the nitrite ligand is coordinated to the manganese atom. The remaining nitrogen atom of the nitrite ligand is uncoordinated. The five ammonia ligands are coordinated to the manganese atom.
Structural formula:
NH3
|
Mn -- O
|
NH3
|
NH3
|
NH3
|
NO2
These representations illustrate the two possible linkage isomers of [Mn(NH3)5(NO2)]2+.
Know more about coordination isomerism here:
https://brainly.com/question/28191611
#SPJ11
How much water should be added to 12 ml of alcohol to obtain 12% alcohol solution?
Answers
Answer:
88 mL of water.
Explanation:
Data obtained from the question include the following:
Volume of alcohol = 12 mL
Percentage of alcohol = 12%
Volume of water =..?
We can thus, obtain the volume of water needed to make 12% of the alcohol solution as follow:
Percentage of the solution = 100%
Percentage of alcohol = 12%
Percentage of water =?
Percentage of water = percentage of solution – percentage of alcohol
Percentage of water = 100 – 12
Percentage of water = 88%
Since 12% of alcohol is equivalent to 12 mL of alcohol, therefore 88% of water will also be equivalent to 88 mL.
Therefore, 88 mL of water is needed to make 12% of alcohol.
Select the curve that is produced by adding hydrochloric acid to 25 cm3 of sodium hydroxide.A,B,C or D

Answers
B
The sodium hydroxide (NaOH) solution is a basic solution, so the pH of that solution should be close to 14
then when adding hydrochloric acid (HCl) we start to neutralice the solution, meaning the pH must sift slowly to lower pH.
Assuming both solutions have similar concentration the pH shall shift form basic (above 7) to acid pH (below 7). Until now both B and D images agreed with the explanation given. To chose between them we need to remember that HCl is a very strong acid, which means that in solution will get to very acid solutions (very low pH values) which leaves only B as possible answer
The amount of matter in an object is its ____
weight
mass
volume
density
Answers
HELPPPPP ME ASAP I'll give you a thanks and mark you as brainliest and give u points
Suppose that the clay balls model the growth of a planetesimal at various stages during its accretion. Choose the planetesimal that is most likely to pull in debris particle A.

Answers
Answer: 2nd one
Explanation:
PLATO
Which SI units would we use in place of inches, pounds and quarts?
Answers
Answer:
45
Explanation:
yes i need help please
which substances corrode copper?
Answers
Copper corrodes at insignificant rates when used in areas with unpolluted air, non-oxidizing acids, and water. However, it happens more rapidly with the presence of road salt, ammonia, sulfur, oxidizing acids
Acidic substances react with the surface of copper, causing it to tarnish and corrode almost instantly. This corrosion is highly soluble, leading to the presence of toxic copper salts in the food. This is why it is not recommended to use copper vessels for foods high in acidity, such as milk, wine, or vinegar.
A metal forms a compound with the formula . if the compound is 60.86hlorine by weight, what is the identity of ?
Answers
The compound with the formula "XCl" is a metal compound where "X" represents the metal element. If the compound is composed of 60.86% chlorine by weight, it means that 60.86 grams out of every 100 grams of the compound is chlorine.
To determine the identity of the metal "X," we need to consider the molar masses of chlorine and the compound as a whole. The molar mass of chlorine is 35.45 g/mol.
To calculate the molar mass of the compound, we can assume a hypothetical sample of 100 grams. Since 60.86 grams is chlorine, the remaining 39.14 grams must be the metal "X."
Next, we need to determine the number of moles of chlorine and the metal in the compound. To do this, we divide the mass of each element by its molar mass.
Number of moles of chlorine = mass of chlorine / molar mass of chlorine
= 60.86 g / 35.45 g/mol
≈ 1.72 mol
Number of moles of metal "X" = mass of metal "X" / molar mass of metal "X"
= 39.14 g / molar mass of metal "X"
To find the molar mass of the metal "X," we need to consider the relationship between the number of moles of chlorine and the number of moles of the metal in the compound. From the chemical formula "XCl," we can infer that the ratio of moles of chlorine to moles of metal "X" is 1:1.
Therefore, 1.72 mol of chlorine is equal to 1.72 mol of metal "X."
Now, we can calculate the molar mass of the metal "X" using the given mass of the metal and the number of moles:
molar mass of metal "X" = mass of metal "X" / number of moles of metal "X"
= 39.14 g / 1.72 mol
≈ 22.75 g/mol
To know more about metal compound visit:-
https://brainly.com/question/19625880
#SPJ11
based on the definition of a mineral, being a naturally occurring solid, formed by inorganic processes, with a characteristic crystal structure and s specific chemical composition, which materials will be classified as minerals?
Answers
Based on the definition of a mineral, being a naturally occurring solid, formed by inorganic processes, with a characteristic crystal structure and a specific chemical composition, materials that will be classified as minerals are those that meet the given criteria.
What are minerals?A mineral is a naturally occurring, solid, crystalline substance that is inorganic in nature and has a distinct chemical composition. Minerals are typically formed by geological processes that occur over a long period of time. Minerals are composed of atoms and molecules that are arranged in a regular, three-dimensional pattern, known as a crystal structure. Because minerals are formed under specific conditions, they have distinct physical and chemical properties.
There are numerous minerals in the earth's crust, and they can be identified based on their physical and chemical properties. Mineralogists study minerals and their properties in order to better understand the earth's composition and history.
Mineral resources are commonly used for industrial purposes such as construction, fuel, and electronics.
Learn more about minerals on:
https://brainly.com/question/15844293
#SPJ11
What is meant by atomic number?
A. number that represents the number of electrons in the valence shell of an atom
B. number that represents how many neutrons are in an atom’s nucleus
C. number that represents how many protons are in an atom’s nucleus
D. number that represents the total number of particles in an atom’s nucleus
Answers
Atomic number of an atom is a number that represents how many protons are in an atom’s nucleus.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
which of the following is an indicator of a chemical reaction?
A. two different compounds is an indicator of a chemical reaction.
B. changing states of matter (solid to liquid)
C. decreasing in size.
D. increasing in temperature
Answers
Answer:
B changing states of matter (solid to liquid)
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
A potassium atom's ground state electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s1.
What substance is electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1?An atom's electron configuration is a picture of how electrons are arranged in relation to orbital shells and subshells. Consequently, this is potassium's electron configuration.
How can you express a whole electron configuration in writing?Making Electron Configurations in Writing. Write the energy level (the period) first, then the subshell that needs to be filled, and finally the superscript, which indicates how many electrons are in that subshell. The atomic number, Z, is the sum of all the electrons.
To know more about electron configuration visit:-
https://brainly.com/question/29757010
#SPJ4
what is the relevant reaction that occurs when a solution of strong acid is added to a buffer comprised of a weak acid (ha) and weak base (a-)?
Answers
The relevant reaction that occurs when a solution of strong acid is added to a buffer comprised of a weak acid (HA) and weak base (A⁻) is the reaction between the strong acid and the weak base.
When a strong acid is added to the buffer, it reacts with the weak base (A⁻) in the buffer. This reaction results in the formation of the conjugate acid of the weak base (HA) and H⁺ ions. The H⁺ ions that are produced in the reaction are then consumed by the weak acid (HA) in the buffer to form more A⁻ ions and maintain the buffer's pH.
In summary, when a solution of strong acid is added to a buffer comprised of a weak acid and weak base, the relevant reaction that occurs is the reaction between the strong acid and the weak base. This reaction results in the formation of the conjugate acid of the weak base and H⁺ ions, which are then consumed by the weak acid in the buffer to maintain the pH of the buffer.
To know more about buffer, visit:
https://brainly.com/question/22821585
#SPJ11
What are the half-reactions for a galvanic cell with Ni and Mg electrodes?
A. Ni(s) → Ni2+(aq) + 2e and Mg2+(aq) + 2e → Mg(s)
B. Ni2+(aq) + 2e → Ni(s) and Mg(s) – Mg2+(aq) + 2e
C. Ni(s) Ni2+(aq) + 2e and Mg(s) → Mg2+(aq) + 2e
D. Ni2+(aq) + 2e → Ni(s) and Mg2+ (aq) + 2e → Mg(s)
Answers
half-reactions
cathode(reduction) : Ni2+(aq) + 2e → Ni(s)
anode(oxidation) : Mg (s) → Mg²⁺ (aq) + 2e−
Further explanationGiven
Ni and Mg reaction
Required
the half-reactions
Solution
we determine which is the more reduced / oxidized of the two elements by looking at the voltaic series or the standard potential value
In voltaic series
Li-K-Ba-Ca-Na-Mg-Al-Mn- (H2O) -Zn-Cr-Fe-Cd-Co-Ni-Sn-Pb- (H) -Cu-Hg-Ag-Pt-Au
The more to the left, the metal is more reactive (easily release electrons) and the stronger reducing agent
The more to the right, the metal is less reactive (harder to release electrons) and the stronger oxidizing agent
If we look at the Mg metal located to the left of the Ni metal, so the Mg metal is more easily oxidized and can reduce the Ni metal which is on the right
Mg oxidation
Mg(s) – Mg2+(aq) + 2e
Ni reduction
Ni2+(aq) + 2e → Ni(s)
which is the strongest base in aqueous solution? a. hoc2h4oh b. ch3oh c. naoh d. nh3
Answers
Answer: option c) the strongest base in aqueous solution is NaOH
Explanation:
the strongest base in aqueous solution is NaOH because strength of a base is determined by its ability to donate hydroxide ions (OH-) in solution. and NaOH dissociates completely in water to produce Na+ and OH- ions. The presence of a fully dissociated hydroxide ion makes NaOH a strong base.
While, HOC2H4OH and CH3OH are weak acids. HOC2H4OH is ethylene glycol and CH3OH is methanol are weak acid due to the presence of the (-OH) group.
Also, NH3 (ammonia), is a weak base though it can accept H⁺ to form NH4+
what does new substances often have that are different from the reactants
Answers
Answer
The new substances often have different combinations of atoms different from the reactants.
Explanation
The reactants and the new substances in a chemical reaction contain the same atoms, but they are rearranged during the reaction. As a result, the atoms end up in different combinations in the new substances. This makes the products new substances that are chemically different from the reactants.
A calculation based upon _______is only as accurate as the device used for measurement.
Answers
hich of the following are aqueous suspensions?
Question 3 options:
Milk
Soil
Paint
Jelly beans
Answers
Use VSEPR theory to predict the bond angles in a molecule of methane (CH4), which is a covalently bonded molecule.
Answers
Answer:
109.5 Degrees
Explanation:
Because CH4 is sp4 hybridized (Carbon is bonded to the 4 Hydrogens), the geometry of the molecule must be tetrahedral. Tetrahedral moleculues have bond angles of 109.5 Degrees.
What is enthalpy change and volume change of mixing of two components forming an ideal solution?
Answers
Enthalpy change of mixing refers to the energy change during the formation of an ideal solution. Volume change of mixing relates to the change in volume resulting from the mixing process.
What is enthalpy ?Enthalpy is a thermodynamic property that represents the total heat content of a system at constant pressure. It encompasses both the internal energy of the system and the work done by or on the system. Enthalpy is denoted by the symbol "H" and is typically measured in units of energy, such as joules (J) or calories (cal). Enthalpy accounts for the energy transferred as heat during chemical reactions or phase changes. Enthalpy is crucial in studying and analyzing various phenomena, including chemical reactions, phase transitions, and energy transfers in thermodynamic systems.
Volume change of mixing, on the other hand, relates to the change in volume resulting from the mixing process. It accounts for the variation in molecular interactions and the resulting effects on the overall volume of the mixture compared to the volumes of the individual components.
To learn more about enthalpy
https://brainly.com/question/14047927
#SPJ4
What is the average atomic mass of
the element in the data table?
Mass (amu)
38.96
39.96
40.96
Abundance (%)
93.26
0.01
6.73
[?]amu
![What is the average atomic mass ofthe element in the data table?Mass (amu)38.9639.9640.96Abundance (%)93.260.016.73[?]amu](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/ZnSSS1JEgfg1fIpVlq9U62m71gNXuvvo.png)
Answers
Answer:
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance.
You discovered a new compound with the following formula: H(O) Pe. Using the information below, what would be the correct name for this compound?
*H(O) is an Alkaline Earth Metal.
*Pe is classified as a Halogen.
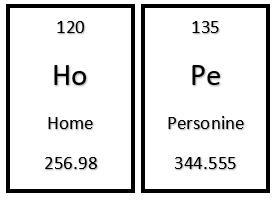
Answers
Answer:
Home personide.
Explanation:
In relation of Magnesium and Chlorine, MgCl2 is called Magnesium chloride
what mass of a 0.1500% solution can be made from 0.2755 g of potassium iodide? b. a 750. ml bottle of merlot is 185 ppm in sodium sulfite. if the density of the wine is 0.992 g/ml, how many grams of sodium sulfite are in the bottle of merlot? c. a 375 ml solution is 0.486 m in sodium acetate. how many grams of sodium acetate are in the solution? d. how would you prepare 1500. g of a 2.000% sucrose (sugar) mass percent solution to be used for intravenous feeding in a hospital?
Answers
10 g of glucose present in 100g of water.
A hospital is a medical facility that provides patient care using specialized medical and medical support staff and medical equipment.
Hospitals and facilities are constructed, staffed, and equipped for the diagnosis of disease. For medical and surgical treatment of the sick and injured. And for their accommodation during this process. Modern hospitals often also serve as a testing and teaching centers.
Hospitals complement and enhance the effectiveness of many other parts of the healthcare system by providing ongoing services for both acute and complex illnesses. They focus scarce resources on a well-planned referral network to efficiently meet the health needs of the population.
Learn more about the hospital here:-https://brainly.com/question/1908877
#SPJ4
How many Particles of silver will be formed from 17.54 g copper, when Copper (II) reacts with Silver Nitrate?
Equation: Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Answers
59.60grams particles of silver will be formed from 17.54 g copper, when Copper (II) reacts with Silver Nitrate. The equation is Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂ (aq) + 2Ag(s).
What is molar mass ?The term molar mass is defined as the mass in grams of one mole of the compound.
The equation is as follows:
Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂ (aq) + 2Ag(s).
1mol of Cu will produce 2 mol Ag.
The molar mass of Cu = 63.5g/mol
The molar mass of Ag = 107.9g/mol
63.5g Cu will produce 2 × 107.9 = 215.8g of Ag
17.54g Cu will produce 17.54g Cu / 63.5gCu × 215.8g Ag
= 0.276 × 215.8
= 59.60grams of Ag produced.
Thus, 59.60grams particles of silver will be formed from 17.54 g copper, when Copper (II) reacts with Silver Nitrate.
To learn more about the molar mass, follow the link;
https://brainly.com/question/12127540
#SPJ1
what would be the effect of bringing a negatively charged metal ball near an iron bar
Answers
When the negatively charged metal ball comes in contact with the iron bar, the positive charges of the bar aligns near to the negative charges and they electrostatically attracts results in a static electricity.
What is static electricity?When a negatively charged body is comes in contact with an conductor or an insulator, the random charges gets polarized, where the positive charges of the second object align with the negative charges and there occurs a flow of electron from the negatively charged object. This is called static electricity.
A metal in its neutral state contains a sea of positive ions and free electrons. Here, the when the negatively charged metal ball comes near the iron bar, the iron bar gets polarized.
The positive charges of the iron bar gets polarized near the negative charge of the ball and there occurs an electrostatic force of attraction. Electrons from the negative charged ball tends to flow to the conductive iron bar and leads to a static electricity.
To find more on static electricity, refer here:
https://brainly.com/question/12791045
#SPJ6
Correct question to answer : Wine goes bad soon after opening because the ethanol CH3CH2OH dissolved in it reacts with oxygen O2 gas to form water and aqueous acetic acid CH3COOH, the main ingredient in vinegar. Calculate the moles of ethanol needed to produce 0.900mol of acetic acid. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.Do not answer : Ammonium phosphate ((NH4)3(PO4) is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of ammonia needed to produce of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
• The balanced equation for the reaction is given by:
CH3CH2OH + O2 → CH3COOH+ H2O• From the above reaction we can see that:
1 mole of ethanol (CH3Ch2OH) produces 1 mol of acetic acid(CH3COOH)
so . x mole of ethanol will produce 0.9mol of acetic acid ....(cross multiply)
xmol ethanol * 1mol acetic = 1mol ethamol* 0.9molacetic
∴ xmol ethanol = 1*0.9 /1
= 0.90 mol
• This means that, 0.90 mol of ethanol, is needed to produce 0.9mol acetic acid,.
What is the mass of 1.6x10^20 molecules of carbon monoxide?
Answers
We need to find the mass of 1.6x10^20 molecules of carbon monoxide
First, we must find the number of mol in the 1.6x10^20 molecules
For this, we need to use the avogadro's number ( 6.02*10^23 )
\(1.6\cdot10^{20}\cdot\frac{1\text{mol}}{6.02\cdot10^{23}}=2.66\cdot10^{-4}\text{mol}\)Then, we must calculate the molar mass of carbon monoxide
M(CO) = 12 g/mol + 16 g/mol = 28 g/mol
Finally, we must multiply the number of mol by the molar mass to find the mass
\(2.66\cdot10^{-4}mol\cdot28\frac{g}{mol}=7.448\cdot10^{-3}g=0.007448g\)ANSWER:
0.007
If an atom has 15 protons and how many electrons will be gained when this atom forms an ion?
Answers
Answer:
What are the Answer choices
Nate and Clara are drawing pictures with markers. There are 8 markers in a set. Nate has 9 markers and Clara has 7. What can Nate and Clara do so that each of them has a full set?
Answers
9-1=8
7+1=8