select the strongest acid from the following list. group of answer choices hbro hclo3 hbro2 hclo2 hio
Answers
The strongest acid in the list is b. HClO3, also known as chloric acid.
To select the strongest acid from the list provided, which includes a. HBrO, b. HClO3, c. HBrO2, d. HClO2, and e. HIO, we can compare their acidic strengths:
To determine acidic strength, we can look at the pka values of each option:
pka value for HBrO = 8.55
pka value for HClO3 = -1.00
pka value for HBrO2 = 4.92
pka value for HClO2 = 7.60
pka value for HIO = 11
Lower the pka value, more is the acidic strength and hence , stronger the acid is. From above written values, we can infer that the lowest pka value is -1.00 and that corresponds to HClO3 i.e, Chloric Acid.
Hence, the strongest acid in the list is b. HClO3, also known as chloric acid.
Learn more about Chloric acid : https://brainly.com/question/30398438
#SPJ11
Related Questions
One idea for avoiding a catastrophic collision of space debris with Earth is the use of missile-like projectiles to knock the object off course or deflect it. How can scientists be sure to create an impact with enough kinetic energy to change a meteoroid’s course? What factors should they consider? HELP :,)
Answers
Describe the molecular structure of the three main types of lipids
Brainliest!!!!!
Answers
Answer:
Lipids are divided into three categories.
Triglycerides, phospholipids, and sterols are the three major forms of lipids.
lead has an atomic number of 82 how many protons and electrons does lead have
Answers
Lead has 82 protons and 82 electrons.
How many protons and electrons are present in a lead atom with an atomic number of 82?Lead, with an atomic number of 82, indicates the number of protons in its nucleus. Therefore, lead has 82 protons. Since atoms are electrically neutral, the number of electrons in an atom is equal to the number of protons. Consequently, lead also has 82 electrons.
The protons, located in the nucleus, carry a positive charge, while the electrons, which orbit around the nucleus, carry a negative charge. The equal number of protons and electrons ensures that the overall charge of a lead atom is neutral.
Learn more about atomic number
brainly.com/question/16858932
#SPJ11
CORRWCT ANSWER GETS BRAINLIST

Answers
Answer:
Parts per million - Option B
Explanation:
Is it possible to explain this ?
Hope this helped and have a good day
Answer: b parts per million
Explanation:
I will make you brainless answer this 1 question pleaaeeee!
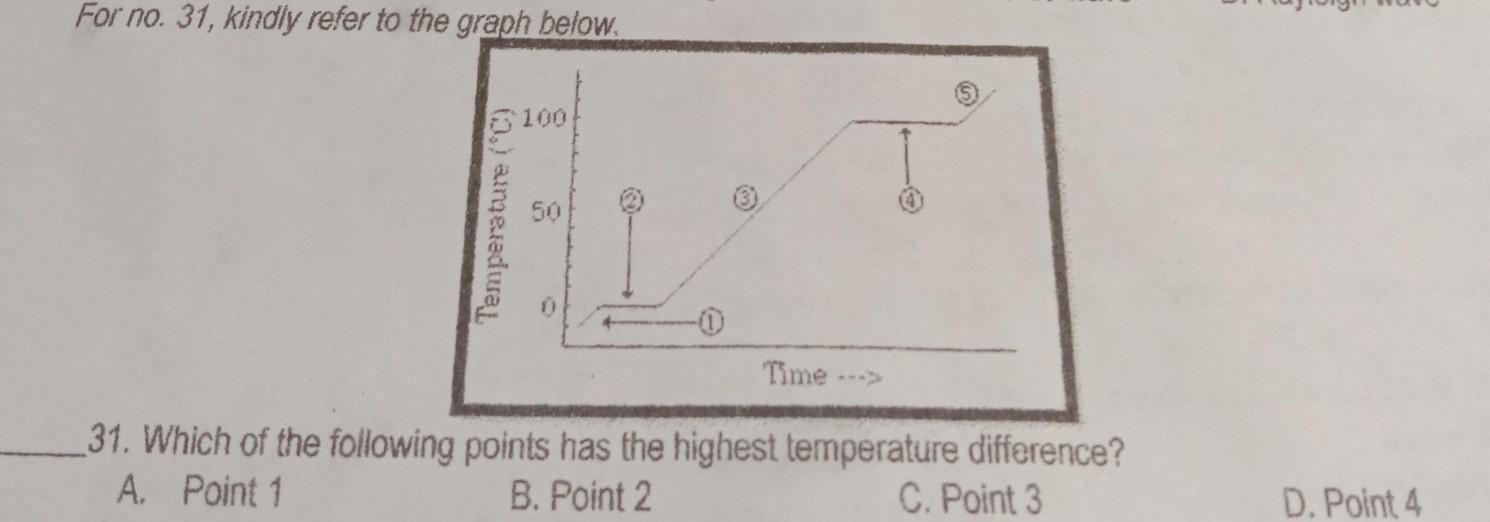
Answers
C. Point 3
Explanation:
Because it is increasing dramatically
Kayla learned that when you play with the Soccket for 30 minutes it gives you 3 hours of light. She thinks that rolling the ball slowly with her baby brother for 30 minutes will give her the same amount of energy as kicking it in a game of soccer for 30 minutes. Do you agree or disagree? State your claim and use evidence from what you've learned about speed and energy to explain your answer.
Answers
Answer:
disagree it wont be the same momentum
Explanation:
cuh
1. What is the definition of a chemical property?
Answers
Answer:
that can be established only by changing a substance's chemical
Explanation:
Answer:
A chemical property is a characteristic of a substance abserved in chemical reactions.
Explanation:
Some major chemical properties include flammability, toxicity, heat of combustion, pH value, rate of radioactive decay, and chemical stability.
How many grams of H2O will be produced if 750. grams of Fe are produced?
Fe3O4 + 4H2 - 3Fe + 4H20
Answers
Answer:
\(\boxed{\small \sf \: Mass \: of \: H_2O =322.2 \: grams}\)
Explanation:
Given:
Mass of ferous (Fe) produced = 750 gram.
To find:
Mass of water produced= ?
Solution:
Molar mass of Fe is 55.84 gram/mol
Let's find out the number of moles of ferous produced.
\( \small \sf Number \: of \: moles = \frac{Given \: mass \: of \: substance }{Molar \: mass \: of \: substance} \)
Substituting the given data in above formula.
\( \small \sf Number \: of \: moles = \frac{750}{55.84} \)
\( \boxed{\small \sf Number \: of \: moles \: of \: Fe= 13.43 \: moles}\)
Now the given reaction is,
\(Fe_3O_4 + 4H_2 \rightarrow 3Fe + 4H_2O\)
For every 3 mole of production of Fe, 4 mole of waters are produced. let for 13.43 moles of Fe x moles of H2O will be produced.now calculate the number of moles of H2O
\( \sf \: \frac{3}{4} = \frac{13.43}{x} \\ \sf x = \frac{13.43 \times 4}{3} \\ \sf x = 17.90 \: moles\)
\( \small \boxed{\sf number \: of \: moles \: of \: H_2O= 17.90 \: moles}\)
mass of one mole of H2O is 18 gram, so we can calculate mass of 17.90 moles.
\(\small \sf \: Mass \: of \: H_2O = 17.90 \times 18 \\ \boxed{\small \sf \: Mass \: of \: H_2O =322.2 \: grams}\)
Thanks for joining brainly community!
a normal copper atom contains 29 electrons. according to the bohr model of the atom, the placement of these electrons, starting with the first shell and moving outward, would be
Answers
According to the Bohr model of the atom, the placement of electrons, in a normal copper atom is as follows: it has 2 eletrons in the first K-shell, 8 in the second L-shell, 19 in the third M-shell.
According to the Bohr model of the atom, electrons are arranged in shells around the nucleus, with each shell being designated by a principal quantum number (n). The first shell, or the K-shell, can hold up to 2 electrons. The second shell, or the L-shell, can hold up to 8 electrons, and the third shell, or the M-shell, can hold up to 18 electrons.
For copper, the first 29 electrons are arranged in the following way:
the first 2 electrons fill the K-shell
the next 8 electrons fill the L-shell
the last 19 electrons fill the M-shell
You can learn more about Bohr model at
https://brainly.com/question/4138548
#SPJ4
Some kids are playing with a jump rope. they notice that they can make transverse waves when they shake the rope up and down. they shake it quickly and notice that the wave that forms appears to be holding still. what type of wave interaction is this?
Answers
The type of wave interaction in this scenario is known as standing waves. When the kids shake the jump rope up and down quickly, they create transverse waves that propagate through the rope.
However, if the frequency of the shaking matches the natural frequency of the rope, a special type of wave called a standing wave is created. In a standing wave, the wave appears to be holding still because it is actually a result of two waves traveling in opposite directions and interfering with each other. This creates points along the rope where the displacement of the wave is always zero, known as nodes, and points where the displacement is at a maximum, known as antinodes.
Standing waves are formed when two waves with the same frequency, amplitude, and wavelength, but traveling in opposite directions, interfere with each other. In this case, when the kids shake the jump rope up and down quickly, they create a wave that travels along the rope and reflects back from the fixed end. The reflected wave then interferes with the incoming wave, creating a standing wave. In a standing wave, some points called "nodes" remain stationary, while other points called "antinodes" oscillate with maximum amplitude.
To know more about transverse waves visit :
https://brainly.com/question/13863548
#SPJ11
What is the main reason that atoms lose or gain electrons to become ions?
Answers
Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations. Most metals become cations when they make ionic compounds.
Ordered: 1000mL of 0.45%NaCl IV for 3 hours Drop factor: 20gt(t)/(m)L Flow rate: gt(t)/(m)in
Answers
The flow rate for the given IV order is 111.2 gt(t)/(m)in.
To calculate the flow rate for the given IV order, we'll use the formula:
Flow rate (gt(t)/(m)in) = Volume (mL) / Time (min)
Given information:
Volume = 1000 mL
Time = 3 hours = 180 minutes
Using the drop factor, we can convert the flow rate from mL/min to gt(t)/(m)in:
Flow rate (gt(t)/(m)in) = Flow rate (mL/min) × Drop factor
To calculate the flow rate (mL/min), we divide the volume by the time:
Flow rate (mL/min) = Volume (mL) / Time (min)
Let's calculate the flow rate step by step:
Flow rate (mL/min) = 1000 mL / 180 min = 5.56 mL/min
Now, we can calculate the flow rate in gt(t)/(m)in by multiplying it by the drop factor:
Flow rate (gt(t)/(m)in) = 5.56 mL/min × 20 gt(t)/(m)L = 111.2 gt(t)/(m)in
To know more about flow rate
brainly.com/question/19863408
#SPJ11
What organ in this system absorbs the nutrients into the blood stream?
Stomach
Small Intestine
Large Intestine
Mouth
Answers
Answer:
SMALL INTESTINE
Explanation:
Answer:
Small Intestine
Explanation:
do ionic compounds or covalent compounds generally have weaker forces of attraction between the ions or molecules making up the substance?
Answers
Explanation:
ionic compunds ............
The efficiency ratio for a steel specimen immersed in a phosphating tank is the weight of the phosphate coating divided by the metal loss (both in mg/ft 2
). Table Q4 gave the accompanying data on tank temperature (x) and efficiency ratio (y). (a) Construct a scatter diagram to represent the relationship between temperature (x) and efficiency ratio (y) on a graph paper. (4 marks) (b) Analyze the relationship between these two parameters by computing the linear regression equation using least square method. (13 marks) (c) Find the correlation coefficient and coefficient of determinations.
Answers
The scatter diagram representing the relationship between temperature (x) and efficiency ratio (y) is constructed on a graph paper using the given data.
How can the scatter diagram be constructed?To construct the scatter diagram, plot the temperature values on the x-axis and the corresponding efficiency ratio values on the y-axis. Each data point will represent a temperature-efficiency ratio pair.
Connect the points to visualize the pattern or trend between the variables. The scatter diagram helps in understanding the nature of the relationship between temperature and efficiency ratio.
Learn more about scatter diagrams
brainly.com/question/30160555
#SPJ11
Al(s) + HCl(aq)→AlCl3(aq) + H2(g) Identify the reactants for this reaction
Answers
Answer:
Al and Hcl
Explanation:
Reactants are the elements, compounds etc before the arrow
A student in the chemistry lab heated a sample of potassium chlorate solid KCIO, (M=122.55 g/mol). 3 The following reaction took place: 2 KClO 2 KCl +30₂ The oxygen gas (M=32 g/mol) produced was collected at 22 °C and 0.964 atm and of vasume 0.65 L. The mass of KCLO 3 that was decomposed in the above reaction is 0.83 2.12 3.17 28.37 grams.
Answers
The mass of KClO₃ that was decomposed in the given reaction by the ideal gas equation is approximately 7.19 grams.
Given:
Pressure (P) = 0.964 atm
Volume (V) = 0.65 L
Temperature (T) = 22 °C = 22 + 273.15 = 295.15 K
The ideal gas law: PV = nRT
Where:
P = Pressure in atm
V = Volume in liters
n = Number of moles
R = Ideal gas constant = 0.0821 L·atm/(mol·K)
T = Temperature in Kelvin
n = (PV) / (RT)
n = (0.964 atm) × (0.65 L) / (0.0821 L·atm/(mol·K) × 295.15 K)
n ≈ 0.0294 mol
2 moles of KClO₃ produce 1 mole of O₂. Therefore, the number of moles of KClO₃decomposed would be:
Moles of KClO₃= 2 × 0.0294 mol
Moles of KClO₃≈ 0.0588 mol
Molar mass of KClO₃= 122.55 g/mol
Mass of KClO₃= Moles of KClO₃× Molar mass of KClO₃
Mass of KClO₃≈ 0.0588 mol × 122.55 g/mol
Mass of KClO₃≈ 7.19 grams
Learn more about ideal gas equation, here:
https://brainly.com/question/15379358
#SPJ1
If you evaporated 125 mL of a 3.5 M solution of iron(II) nitrite, how many moles of iron(II) nitrite would you recover?
Answers
If 125 mL of a 3.5 M solution were evaporated, 0.438 moles of iron (II) nitrite would be recovered.
What is molarity?Molarity is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of moles of solute per liter of solution.
We want to find the moles of iron (II) nitrite (solute) in 125 mL of a 3.5 M solution. We will use the definition of molarity.
M = n / V(L)
n = M × V(L) = 3.5 mol/L × 0.125 L = 0.438 mol
where,
M is the molarity.n is the number of moles.V(L) is the volume of the solution in liters.If 125 mL of a 3.5 M solution were evaporated, 0.438 moles of iron (II) nitrite would be recovered.
Learn more about molarity here: https://brainly.com/question/9118107
A reaction that had two compounds as reactants and two compounds as products is most likely a
Answers
double-replacement reaction
what differentiates two isotopes of a given element?
Answers
Two isotopes of any particular element differs on the count of number of neutrons present on its nucleus.
Isotopes are particular atomic species (or nuclides, as specialized term) of a similar component. They have a similar nuclear number (number of protons in their cores) and position in the occasional table (and subsequently have a place with a similar synthetic component), however contrast in nucleon numbers (mass numbers) because of various quantities of neutrons in their cores. While all isotopes of a given component have practically similar substance properties, they have different nuclear masses and actual properties.
The term isotope is framed from the Greek roots isos and topos , signifying "a similar spot"; consequently, the importance behind the name is that various isotopes of a solitary component possess a similar situation on the periodic table. It was begat by Scottish specialist and essayist Margaret Todd in 1913 in an idea to the English scientist Frederick Soddy.
To know more about isotopes,visit here:
https://brainly.com/question/12955625
#SPJ4
A scientist discovers an object in the solar system. She describes it as bigger
than an asteroid, smaller than Mercury, and farther from the sun than Neptune.
What kind of object could it be? Explain.
Answers
Answer:
Pluto I think it's the ans
Which one of the following Lewis structures is definitely incorrect? 1) A. BF; B. XeO C. Ne D. AICI, E. NH
Answers
Option A, BF, does not violate the octet rule, option B, XeO, satisfies the octet rule for all atoms, option C, Ne, is a noble gas and already has a complete octet, and option D, AICI3, has complete octets for both the atoms and does not violate any rules.
The incorrect Lewis structure is likely to be the one that violates the octet rule, has an incomplete octet or has an odd number of electrons.
In the given options, only option E, NH, violates the octet rule. Nitrogen has five valence electrons and each hydrogen has one valence electron. If we draw the Lewis structure for NH, we get three lone pairs on nitrogen and one unpaired electron.
This makes a total of nine valence electrons, which is one more than the total available. Therefore, NH does not follow the octet rule and is the incorrect Lewis structure.
Visit here to learn more about atoms:
brainly.com/question/6258301
#SPJ11
What is the molecular formula if the empirical formula is CH2O and the molecular molar mass is 180.18?
Answers
Answer:
The given chemical compound has 2 atoms of hydrogen and one atom of oxygen for each atom of carbon. The mass of CH2O is 12 + 2*1 + 16 = 30. The molecular weight of the compound is 180.18 which is approximately 180. This gives the molecular formula of the chemical compound as C6H12O6.
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
During mitosis, this structure moves individual chromosomes?
Answers
Answer:
Anaphase
Explanation:
Two separate classes of movements occur during anaphase. During the first part of anaphase, the kinetochore microtubules shorten, and the chromosomes move toward the spindle poles. During the second part of anaphase, the spindle poles separate as the non-kinetochore microtubules move past each other.
Which area on the illustration represents the largest reservoir of nitrogen on earth?.
Answers
The largest reservoir of nitrogen on earth is represented by the atmosphere in the illustration. Nitrogen is a gas that makes up approximately 78% of the earth's atmosphere. It is a major component of the atmosphere and therefore, the largest reservoir of nitrogen is located in the atmosphere.
Other reservoirs of nitrogen include soil, oceans, and living organisms. However, the amount of nitrogen in these reservoirs is much smaller than the amount in the atmosphere. Nitrogen is found in various reservoirs on Earth, including the atmosphere, soil, plants, and water bodies. However, the atmosphere contains approximately 78% of nitrogen gas (N2), making it the largest reservoir of nitrogen on Earth.
Nitrogen is essential for life, as it is a key component of amino acids and nucleic acids, which are the building blocks of proteins and DNA, respectively. Step by step explanation, 1. Identify the various reservoirs of nitrogen atmosphere, soil, plants, and water bodies. 2. Determine the percentage of nitrogen present in each reservoir. 3. Compare the percentages and conclude that the atmosphere, with approximately 78% nitrogen gas (N2), is the largest reservoir of nitrogen on Earth.
To know more about nitrogen visit:
https://brainly.com/question/16711904
#SPJ11
Determine the number of significant figures in the following measurement: 32.06 mL
Answers
Answer:
There are four significant figures
Explanation:
There are four digits within 32.06, 3, 2, 0, and 6. Significant figures are the number of digits included in a number (besides the 0 in 0.xxx), therefore, there are four significant figures :)
describe where
the energy is coming from and how it is affecting change or putting an object into motion
help me and fast

Answers
The energy is coming from the battery that is in the torchlight.
In the case of an object in motion, energy is coming from the kinetic energy of the object. Kinetic energy is the energy an object possesses due to its motion. This energy is transferred to the object by an external force, such as a push or a pull, which causes the object to start moving or to change its motion.
What is the energy about?Energy is the ability to do work and can take many forms, such as thermal, kinetic, potential, and chemical energy. Energy can be transformed from one form to another and can also be transferred from one object to another.
Therefore, In the case of an object being put into motion, energy is coming from the potential energy of the object. Potential energy is the energy an object possesses due to its position or configuration. This energy is transferred to the object by an external force, such as gravity, which causes the object to start moving or to change its motion.
Learn more about energy from
https://brainly.com/question/2003548
#SPJ1
How much heat is released when 24.8 g of ch4 is burned in excess oxygen gas?
Answers
The given question is incomplete. The complete question is:
How much heat is produced when 24.8 g of \(CH_4\) is burned in excess oxygen gas
Given: \(CH _4 +2O_2\rightarrow CO_2+2H_2O\) ΔH= −802 kJ.
Answer: 1243.1 kJ
Explanation:
Heat of combustion is the amount of heat released on complete combustion of 1 mole of substance.
Given :
Amount of heat released on combustion of 1 mole of methane = 802 kJ kJ/mol
According to avogadro's law, 1 mole of every substance occupies 22.4 L at NTP, weighs equal to the molecular mass and contains avogadro's number \(6.023\times 10^{23}\) of particles.
1 mole of \(CH_4\) weighs = 16 g
Thus we can say:
16 g of \(CH_4\) on combustion releases heat = 802 kJ
Thus 24.8 g of \(CH_4\) on combustion releases =\(\frac{802}{16}\times 24.8=1243.1kJ\)
Thus heat released when 24.8 g of methane is burned in excess oxygen gas is 1243.1 kJ
Answer either or both questions please!


Answers
Answer:
Picture 1: Unhealthy
Picture 2: Healthy
Explanation:
Too much algae can start releasing toxins into the air burning the ozone hole and affecting climate change
Invertebrates are healthy, like bees who make honey for us., In truth most bugs we need in this world, even if we don't notice them
Brainliest please