Answers
Answer:
c
Explanation:
litmus +magnisiumSodium
Li+MgS
What type of reaction is this?
O Synthesis
O Combustion
O Double Replacement
O Single Replacement
C 3
Related Questions
what is the chemical name of burning
Answers
Answer:
Combustion is commonly called burning, and the substance that burns is usually referred to as fuel. The products of a complete combustion reaction include carbon dioxide (CO2) and water vapor (H2O).
What data would be most helpful to support the claim that the population of a certain plant is dependent on the rainfall in its habitat?
OPTIONS
The average amount of water absorbed by the roots and given off by the leaves of these plants
The amount of water retained in the soil in the area where the plants live
Rainfall amounts and number of these plants in a certain habitat over several years
The heights and number of leaves produced by these plants under different moisture conditions
Answers
Explanation:
make up some of your own signs which can warns people about the dangers of river an ocean a large snake hiding in the bush
There are a number of factors that alter the bioavailability of minerals from foods. Complete each sentence with either increases or decreases.
decreases increases
Phytic acid ______ the absorption of zinc from grains.
Oxalic acid _____ the absorption of calcium from spinach.
Refinement of grains _____ the mineral content of grain products.
Vitamin C _____ the absorption of iron from foods.
Vitamin D ____ the absorption of calcium from foods.

Answers
Each sentence about the bioavailability of minerals in foods should be completed with either increases or decreases as follows:
Phytic acid decreases the absorption of zinc from grains.Oxalic acid decreases the absorption of calcium from spinach.Refinement of grains decreases the mineral content of grain products.Vitamin C increases the absorption of iron from foods.Vitamin D increases the absorption of calcium from foods.What are nutrients?Nutrients can be defined as the chemical compounds (substances) or minerals that are present in food, and they are needed by the body of a living organism for healthy growth, reproduction, development, proper functioning, and reproduction.
What are the classes of nutrients?In Science, there are five (5) main classes of nutrients and these include the following:
ProteinFatCarbohydrateVitaminsMineralsIn conclusion, phytic acid, oxalic acid, and refinement of grains are factors that decrease the bioavailability of minerals in foods while Vitamin C and Vitamin D increases the bioavailability of minerals in foods.
Read more on nutrients here: brainly.com/question/4413413
#SPJ1
Consider the decomposition of water, is energy released or absorbed in this reaction? Discuss bond energy in your answer.!
Answers
Answer:
Then decomposition of water involves its breakdown into Hydrogen and Oxygen
The energy derived is absorbed which means it’s an endothermic reaction. If the energy is released to the surroundings/environment, it is termed as exothermic.
In an endothermic reactions, the reaction occurs when the bonds present in the reactants are broken and greater than the release of new bonds during products formation.
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
Write a decay equation for neon-19
Answers
Answer:
Neon
Mass Number Half-life Decay Mode
Electron Capture
Electron Capture with delayed Proton Emission
18 1.6670 seconds Electron Capture
19 17.22 seconds Electron Capture

Using Newton’s 2nd Law (F = ma) complete the following table for different types of cars.
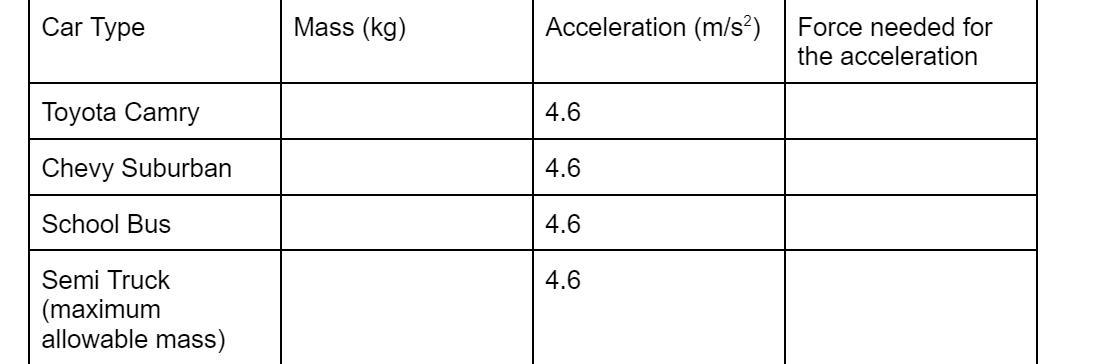
Answers
The force needed to accelerate the Toyota Camry is 7,659 N.
The force needed to accelerate the Chevy Suburban is 11,297.6 N.
The force needed to accelerate the School bus is 83,720 N.
The force needed to accelerate the Semi Truck is 193,701.4 N.
What is Newton's second law of motion?Newton's second law of motion states that the force applied to an object is directly proportional to the product of mass and acceleration of the object.
Mathematically, Newton's second law of motion is given as;
F = ma
where;
m is the mass of the objecta is the acceleration of the objectThe given parameters include
mass of the Toyota Camry, m = 1665 kgmass of Chevy Suburban, m = 2,456 kgmass of school bus, m = 18,200 kgmass of semi truck, m = 42,108 kgThe force needed to accelerate each of the cars is calculated as follows;
F ( Toyota Camry ) = ( 1665 kg ) x ( 4.6 m/s² ) = 7,659 N
F ( Chevy Suburban ) = ( 2,456 kg ) x ( 4.6 m/s² ) = 11,297.6 N
F ( school bus ) = ( 18,200 kg ) x ( 4.6 m/s² ) = 83,720 N
F ( Semi Truck ) = ( 42,109 kg ) x ( 4.6 m/s² ) = 193,701.4 N
Thus, the force needed to accelerate each cars is a function of mass and acceleration of each of the cars.
Learn more about applied force here: https://brainly.com/question/24255032
#SPJ1
The complete question is below;
Using Newton’s 2nd Law (F = ma) complete the following table for different types of cars. mass of the Toyota Camry, is 1665 kg, the mass of Chevy Suburban, m = 2,456 kg, the mass of school bus 18,200 kg and the mass of semi truck, m = 42,108 kg
Is a Krypton atom still a Krypton atom if you change the number of neutrons?
Group of answer choices
Answers
Answer:
Yes
Explanation:
A krypton atom will remain the same despite changing the number of neutrons they contain.
The neutron is a nuclear particle found within an atom.
Atoms with different number of neutrons belonging to the same element are called isotopes. Only changes to the number of protons changes the elemental designation of an atom. A change in the number of electrons and neutrons does not affect the identity of such atom.Which of the following is an example of a situation where work occurs?
A
a glass of water sitting on the counter
B
a leaf resting on a rock
с
a student sitting at a desk
D
a baseball soaring through the air
Answers
Answer:
a baseball soaring through the air
Explanation:
According to the passage, “Work occurs whenever something is moved and an object is put into motion.” A baseball soaring through the air is an object in motion.
An example of a situation where work occurs is a baseball soaring through the air. Therefore, option D is correct.
What is work ?Work is defined as the energy that is applied to or removed from an object by applying force along a displacement. In its simplest form, it equals the product of the force's magnitude and the distance traveled for a constant force directed in the same direction as the motion.
Inertia law. Its name derives from an object's propensity to resist any change in motion up until an out-of-balance force acts on it.
A force that opposes motion is friction. Friction occurs when two moving objects come into touch and operates in the opposite direction.
The angle between force and displacement determines the kind of work that is done. Positive work occurs when a force is applied and an object is moved in that direction.
Thus, option D is correct.
To learn more about work, follow the link;
https://brainly.com/question/19382352
#SPJ2
What is the coefficient of iron(lll) carbonate?
Fe(NO3)3 + (NH4)2CO3
? Fe2(CO3)3 + NH4NO3
Answers
The balanced equation would be 2Fe(NO3)3 + 3(NH4)2CO3 → Fe2(CO3)3 + 6NH4NO3.
Here the question. It's a concentration calculation.

Answers
Answer:
To find the molarity of a 5% vinegar solution, you first need to determine the mass of acetic acid in the solution. To do this, you can multiply the mass of the solution by the mass fraction of acetic acid.
The mass fraction of acetic acid in a 5% vinegar solution is 5%, or 0.05. The mass of the solution is the density of the solution multiplied by the volume of the solution. Since the density of the solution is 1.01 g/mL, you can use the following formula to calculate the mass of the solution:
Mass of solution = density * volume
Once you have the mass of the solution, you can use the following formula to calculate the mass of acetic acid in the solution:
Mass of acetic acid = mass of solution * mass fraction of acetic acid
Once you have the mass of acetic acid, you can use the molar mass of acetic acid to calculate the number of moles of acetic acid in the solution. Finally, you can use the volume of the solution to calculate the molarity of the solution using the following formula:
Molarity = number of moles / volume
I hope this helps! Let me know if you have any questions.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
Write any two drawbacks of the octet theory.
Answers
Answer:
Octet rule fails to explain the following:
(1) The stability of incomplete octet molecules, i.e., the molecules with the central atom containing less than eight electrons. (2) The stability of expanded octet molecules, i.e., the molecules with the central atom containing more than eight electrons.
Sally does the work in 2.3 hours and Pete does 2.5
Answers
((Please help asap! Giving brainliest))
Which of the following explains how Albert Einstein tried to influence the use of his discovery of the energy equation? (5 points)
Select one:
a. He opposed the use of nuclear fusion during the Manhattan Project.
b. He recommended uncontrolled fusion for medical testing and treatment.
c. He recommended applying the principles of nuclear fission to defend against enemies.
Answers
B
i hope this helped!
None of the above are correct.
Albert Einstein supported the use of radioactive elements for medical diagnostic purposes.
I hope this helps, good luck!
Answer fast please!!
A calorimeter contains 600 g of water at 25°C. You place a hand warmer containing 300 g of liquid
sodium acetate inside the calorimeter. When the sodium acetate finishes crystallizing, the temperature of
the water inside the calorimeter is 36.4°C. The specific heat of water is 4.18 J/g-°C. What is the enthalpy
of fusion (AH) of the sodium acetate? (Show your work.) Where necessary, use q = mH₁.
Answers
The enthalpy of fusion of sodium acetate is approximately -93.64 J/g.
To calculate the enthalpy of fusion (ΔH) of sodium acetate, we can use the principle of energy conservation. The heat lost by the hand warmer during the crystallization process is equal to the heat gained by the water in the calorimeter.
First, let's calculate the heat gained by the water in the calorimeter using the formula q = m × c × ΔT, where q is the heat gained or lost, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
q_water = m_water × c_water × ΔT_water
Given:
m_water = 600 g
c_water = 4.18 J/g-°C
ΔT_water = 36.4°C - 25°C = 11.4°C
q_water = 600 g × 4.18 J/g-°C × 11.4°C
q_water = 28092 J
Since the heat lost by the hand warmer during crystallization is equal to the heat gained by the water, we can write:
q_water = q_handwarmer
Now, let's calculate the heat lost by the hand warmer using the same formula:
q_handwarmer = m_handwarmer × c_handwarmer × ΔT_handwarmer
Given:
m_handwarmer = 300 g
c_handwarmer = unknown (specific heat capacity of sodium acetate)
ΔT_handwarmer = 36.4°C - initial temperature of sodium acetate
Since the sodium acetate undergoes crystallization, its temperature remains constant during this phase change. The temperature at which crystallization occurs is known as the freezing point of sodium acetate, which is approximately 58°C. Therefore:
ΔT_handwarmer = 36.4°C - 58°C = -21.6°C
Now, we can substitute the known values into the equation:
q_water = q_handwarmer
28092 J = 300 g × c_handwarmer × -21.6°C
To solve for c_handwarmer, we rearrange the equation:
c_handwarmer = -28092 J / (300 g × -21.6°C)
c_handwarmer ≈ 5.47 J/g-°C
The specific heat capacity of sodium acetate (c_handwarmer) is approximately 5.47 J/g-°C.
The enthalpy of fusion (ΔH) can be calculated using the equation ΔH = q_handwarmer / m_handwarmer:
ΔH = -28092 J / 300 g
ΔH ≈ -93.64 J/g
For more such questions on fusion visit:
https://brainly.com/question/3992688
#SPJ8
Which of the following has the highest frequency?
1. radio wave
2. micro wave
3. visible light
4. Ultra violet
Оа
Radio wave
Oь
micro wave
visible light
Od
Ultra violet
Answers
How many moles of CO2 must dissolve in excess water to produce 12 moles of H2CO3?

Answers
Answer:
12 moles of CO₂.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
CO₂ + H₂O —> H₂CO₃
From the balanced equation above,
1 mole of CO₂ dissolves in water (H₂O) to produce 1 mole of H₂CO₃.
Finally, we shall determine the number of mole of CO₂ needed to produce 12 moles of H₂CO₃. This can be obtained as follow:
From the balanced equation above,
1 mole of CO₂ dissolves in water (H₂O) to produce 1 mole of H₂CO₃.
Therefore, 12 moles of CO₂ will also dissolves in water (H₂O) to produce 12 moles of H₂CO₃.
How many moles of MgS are in 1.00g MgS?
Answers
Answer:
24.31 g/mol.
Explanation:
moles =mass/molar mass
n=w/m
What is The Magnus Effect/Force? Explain.
Answers
Answer:
dk
Explanation:
What is the coefficient for sodium chloride when this equation is balanced?
Answers
Answer:
To resolve this, we need to place the coefficient “2” in front of the sodium in the reactant, to give the equation shown below. 2 Na (s) + Cl 2 (g) → 2 NaCl (s) In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced.
Explanation:
Explain how a scanning electron microscope (SEM) can be used in combination with an energy dispersive X-ray spectrometer (EDS) to perform elemental analysis on extremely small particles by placing the statements in the order that they occur.

Answers
Answer:
SEM provides detailed high resolution images of the sample by rastering a focussed electron beam across the surface and detecting secondary or backscattered electron signal. An Energy Dispersive X-Ray Analyzer (EDX or EDA) is also used to provide elemental identification and quantitative compositional information.
Energy Dispersive X-Ray Spectroscopy (EDS) is a chemical microanalysis technique used in conjunction with scanning electron microscopy (SEM).
The EDS technique detects x-rays emitted from the sample during bombardment by an electron beam to characterize the elemental composition of the analyzed volume.The SEM is an instrument that produces a largely magnified image by using electrons instead of light to form an image. A beam of electrons is produced at the top of the microscope by an electron gun. Once the beam hits the sample, electrons and X-rays are ejected from the sample. The electrons in the beam interact with the sample, producing various signals that can be used to obtain information about the surface topography and composition.Learn more:
brainly.com/question/15899795
Are there any structural similarities besides functional groups that might be used to classify
these molecules? If so, what are they?
Answers
Besides functional groups, alkanes can be classified based on structural similarities. One key structural similarity is the carbon skeleton, or backbone, of the molecule. The carbon skeleton refers to the arrangement and connectivity of carbon atoms in the molecule.
Alkanes can be broadly categorized into two types: straight-chain alkanes and branched-chain alkanes. Straight-chain alkanes have a linear arrangement of carbon atoms with no branches or side chains. The carbon atoms are connected in a sequential manner, forming a simple chain. Examples include methane, ethane, propane, and butane. On the other hand, branched-chain alkanes have a non-linear carbon backbone. They possess branches, or side chains, which are additional carbon chains that deviate from the main carbon chain.
Learn more about the alkanes here.
https://brainly.com/question/31386716
#SPJ1
Are there any structural similarities besides functional groups that might be used to classify the alkanes molecules? If so, what are they?
Forensics:
roshni, a forensic scientist, has ruled out several different drugs when testing a substance found in a car. She suspects that the substance is cocsine, what should roshni do next?
1-Perform a confirmatory test
2-Put the substance in a bag, label it, and send it to a DEA office
3-Confer with a colleague about her suspicions.
4-Collect a blood sample and see if it also contains the same substance
Answers
Since she suspects that the substance is cocsine, the thing that she should do next is option 1-Perform a confirmatory test.
How the Evidence Is Collected?The way that Drug evidence is collected is that from any crime scene, evidence is obtained, photographed, packaged, documented as well as been sent for analysis and then the forensic scientist will carry out the Analysis.
Since the evidence has been sent to Roshni, all she has to do is to carry out the confirmatory test to see if the drug is what she suspected.
Therefore, Since she suspects that the substance is cocsine, the thing that she should do next is option 1-Perform a confirmatory test.
Learn more about forensic scientist from
https://brainly.com/question/27111095
#SPJ1
Find the pH of these buffer solutions using the information provided: 1L solution containing 80g of lactic acid (MW
Answers
Answer:
pH of the solution is 2.0
Explanation:
The lactic acid is a weak acid that is in equilibrium with water as follows:
Lactic acid + H2O ⇄ Lactate + H₃O⁺
And Ka for lactic acid: 1.38x10⁻⁴
Ka = 1.38x10⁻⁴ = [Lactate] [H₃O⁺] / [Lactic acid]
Initial concentration of lactic acid is (MW: 112.06g/mol):
80g * (1mol / 112.06g) / 1L = 0.714M
The equilibrium concentration of the species in the equilibrium are:
[Lactate] = X
[H₃O⁺] = X
[Lactic acid] = 0.714-X
Replacing in Ka expression:
1.38x10⁻⁴ = [X] [X] / [0.714-X]
9.8532x10⁻⁵ - 1.38x10⁻⁴X = X²
9.8532x10⁻⁵ - 1.38x10⁻⁴X - X² = 0
Solving for X:
X = -1.0x10⁻². False solution, there is no negative concentrations
X = 9.86x10⁻³M. Right solution.
As [H₃O⁺] = X
[H₃O⁺] = 9.86x10⁻³M
and pH = -log [H₃O⁺] = -log 9.86x10⁻³M
pH = 2.0
pH of the solution is 2.0A scientific law is different from a scientific theory because it describes something in nature without attempting to explain it.
Answers
Yes, that statement is generally correct. A scientific law is a statement that describes a phenomenon or pattern in nature, often expressed mathematically, without attempting to explain why it occurs. A scientific theory, on the other hand, is a well-substantiated explanation for a set of phenomena, based on empirical evidence and scientific reasoning.
A scientific law summarizes what happens in a particular situation, often in the form of an equation or formula, whereas a scientific theory attempts to explain why it happens.
For example, the law of gravity describes the attraction between masses, but it does not explain why this attraction occurs. In contrast, the theory of general relativity attempts to explain the underlying principles of gravity, including its effects on the curvature of space-time.
It's worth noting that both scientific laws and scientific theories are based on empirical evidence, but they serve different purposes in scientific inquiry. Laws describe what happens in a particular situation, while theories attempt to explain why it happens.
For more question on scientific law click on
https://brainly.com/question/16347879
#SPJ11
sodium bicarbonate =sodium carbonate + water + carbon dioxide gas
Answers
Answer: NaHCO3 = Na2CO3 + H2O + CO2
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb
Chemist I found that 24.31 g magnesium combined with 71.33 g chlorine. Chemist II found that
109.4 mg magnesium combined with 319.1 mg chlorine. You found that 3.647 g magnesium
combined with 10.700 g chlorine? How do your results compare to the two chemists? Show your
work
A. You disagree with both chemists.
B. You agree with Chemist I only.
C. You agree with Chemist II only.
D. You agree with both chemists.
Answers
The compound magnesium chloride is produced when magnesium and chlorine interact. Below is a formula for the equation. Mg+Cl2→MgCl2.
To determine accuracy or systematic error, an experiment using different methodologies is conducted.
What is the purpose of comparison in an experiment?If a significant difference exists between an experimental average and the population mean (), also known as the "true value," the t-test is employed to ascertain this. With the help of standard reference materials and quality control standards, this method is used to assess the findings of experiments.
In the range of research techniques currently employed in science, comparative studies play a crucial role. They enable scientists to use a treatment-control design in circumstances where experimentation is not an option, and they can offer priceless knowledge on the relationships between variables.
To determine accuracy or systematic error, an experiment using different methodologies is conducted. To see how this experiment works with the others, review MV - The Experimental Plan.
To learn more about experiment refer to:
https://brainly.com/question/25303029
#SPJ1
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.