Answers
We have that the Initial volume of buret (mL) is give as
\(V_1=(ml)\)From the question we are told
Sample 2: Initial volume of buret (mL)
Initial volumeGenerally In ital volume is a relative terminology as you cant have a initial without having a final
the initial volume of the brute speaks to a gauge of the buret in comparison to another gauge of the buret
From the image the buret has its present gauge at 4.1(mL)
and this might be either the initial or the final
But we ascertain that as the final volume , and as the image looks to start at a gauge of 3(ml)
Therefore
The initial volume of buret is at
\(V_1=(ml)\)For more information on buret visit
https://brainly.com/question/20117360
Related Questions
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more: The wrong wavelength may be set. There may be an issue with the spectrophotometer. The sample may be placed impro
Answers
The question is incomplete; the complete question is;
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more:
The wrong wavelength may be set.
The sample may be placed improperly in the cuvette holder.
There may be an issue with the composition of the sample.
There may be an issue with the spectrophotometer
Answer:
The wrong wavelength may be set.
There may be an issue with the spectrophotometer
Explanation:
Substances do not absorb radiation at all wavelengths. The proper wavelength at which a substance absorbs must be used for a reading to be obtained from the spectrophotometer. If this is not done, no reading is obtained from the spectrophotometer.
Generally, if the spectrophotometer has an issue, it may display no reading until the machine is fixed.
The possible issues if a sample in a spectrophotometer gives no reading are:
The sample may be placed improperly in the cuvette holder. The wrong wavelength may be set.According to the given question, we are asked to identify the possible reasons why a spectrophotometer would give no reading when a sample is used on it.
As a result of this, it is important to note that it is possible that the sample may not have been placed correctly on the cuvette holder or the wrong wavelength may be set which would cause the no reading error to show.
Read more here:
https://brainly.com/question/13998813
why did my dad hasn't come back with the milk for 10 years
Answers
Answer:
Milk's heavy
Explanation:
In the SOLID state of matter ,particles have enough energy to move freely but not enough energy to overcome their attraction for each other
Answers
In the solid state of matter, particles, such as atoms, ions, or molecules, are closely packed and held together by strong intermolecular forces, such as ionic bonds, metallic bonds, or covalent bonds.
In a solid, particles have enough energy to vibrate around fixed positions but do not have enough energy to overcome the attractive forces between them. These attractive forces, also known as cohesive forces, arise from the electrostatic interactions between particles or the sharing of electrons in covalent bonds.
The energy of the particles in a solid is typically much lower than in the liquid or gaseous states, resulting in a fixed arrangement of particles.
The movement of particles in a solid is characterized by vibrations or oscillations around their equilibrium positions.
These vibrations occur due to the thermal energy present in the solid, but the particles remain relatively fixed in their positions due to the strong attractive forces. The amplitude of the vibrations increases with increasing temperature, as the particles gain more thermal energy.
However, the particles in a solid do not have enough energy to break the intermolecular bonds and move freely throughout the entire solid. Instead, they can only move within their local vicinity or lattice positions.
This restricted movement is what distinguishes the solid state from the liquid or gaseous states, where particles have enough energy to overcome intermolecular forces and move more freely.
For more such questions on intermolecular forces visit:
https://brainly.com/question/12243368
#SPJ8
Reaction of 2,3-dimethyl-1-butene with HBr leads to an alkyl bromide, C6H13Br. On treatment of this alkyl bromide with KOH in methanol, elimination of HBr occurs and a hydrocarbon that is isomeric with the starting alkene is formed. What is the structure of this hydrocarbon, and how do you think it is formed from the alkyl bromide
Answers
Answer:
See explanation and image attached
Explanation:
The image attached shows the entire scheme of reactions mentioned in the question.
The first reaction is an addition reaction which yields a tertiary alkyl halide as shown in accordance with Markovnikov rule.
The second reaction is a dehydrohalogenation in which the base abstracts a proton from the alkyl halide followed by loss of a bromide ion to yield the corresponding alkene.
This alkene is an isomer of the starting material.

What is the molecular formula of each of the following
compounds?
(a) empirical formula CH₂, molar mass = 84 g/mol
(b) empirical formula NH₂Cl, molar mass = 51.5 g/mol
Answers
(a) the molecular formula of the compound is C₆H₁₂.
(b) the molecular formula of the compound is NH₂Cl.
(a) Given the empirical formula CH₂ and a molar mass of 84 g/mol, we need to determine the molecular formula. To do so, we need to find the factor by which the empirical formula needs to be multiplied to achieve the given molar mass.
The empirical formula CH₂ has a molar mass of 14 g/mol (12 g/mol for carbon + 2 g/mol for hydrogen).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 84 g/mol / 14 g/mol = 6
Therefore, the molecular formula is obtained by multiplying the empirical formula by the factor:
CH₂ × 6 = C₆H₁₂
Thus, the molecular formula of the compound is C₆H₁₂.
(b) Given the empirical formula NH₂Cl and a molar mass of 51.5 g/mol, we follow a similar approach.
The empirical formula NH₂Cl has a molar mass of 51.5 g/mol (14 g/mol for nitrogen + 2 g/mol for each hydrogen + 35.5 g/mol for chlorine).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 51.5 g/mol / 51.5 g/mol = 1
Therefore, the molecular formula is the same as the empirical formula: NH₂Cl
Hence, the molecular formula of the compound is NH₂Cl.
for more questions on molecular
https://brainly.com/question/24191825
#SPJ8
Pure silver has a brilliant white metallic luster. How many moles of silver, Ag, are in 7.54 × 1023 atoms of silver?
Answers
The number of moles of silver, Ag, that are in 7.54 × 10²³ atoms of silver is 1.25 moles
From the question,
We are to determine the number of moles of silver, Ag, that are present in 7.54 × 10²³ atoms of silver
From the formula
\(Number\ of\ moles =\frac{Number\ of\ atoms}{Avogadro's\ constant}\)
Avogadro's constant = 6.022 × 10²³ atoms mol⁻¹
and
From the given information
Number of silver atoms = 7.54 × 10²³ atoms
Putting the parameters into the formula, we get
Number of moles of silver present = \(\frac{7.54\times 10^{23} }{6.022\times 10^{23}}\)
Number of moles of silver present = 1.2520757 moles
Number of moles of silver present ≅ 1.25 moles
Hence, the number of moles of silver, Ag, that are in 7.54 × 10²³ atoms of silver is 1.25 moles
Learn more on calculating number of moles here: https://brainly.com/question/15839520
What is paper made of?
Answers
Paper used as a writing material is made of pulp (wood).
What is paper?Paper is a sheet material used for writing on or printing on (or as a non-waterproof container), usually made by draining cellulose fibres from a suspension in water.
Paper is made from cellulose found in trees, which are the main source of cellulose fibre (or woodpulp). Besides woodpulp, paper can be made from other materials such as cotton, flax, esparto, straw, hemp, manilla and jute.
Wood pulp is usually a softwood, used for pulping to make paper.
Learn more about pulp at: https://brainly.com/question/23590026
#SPJ1
How does a polar covalent bond differ from a covalent bond
Answers
Covalent bonds involve equal sharing of electrons while polar covalent bonds involve unequal sharing of electrons.
Polar covalent vs covalent bondsA covalent bond involves the sharing of electrons between two atoms. A polar covalent bond is a type of covalent bond where the electrons are not shared equally between the atoms.
This occurs when one atom in the bond has a higher electronegativity than the other, resulting in an unequal distribution of electrons.
The result is a bond with a partial positive and a partial negative charge, creating a polar molecule. In contrast, a nonpolar covalent bond involves an equal sharing of electrons between two atoms.
More on covalent bonds can be found here:https://brainly.com/question/19382448
#SPJ1
Four grams of hydrogen react completely with 32 grams of oxygen. Based on the law of conservation of mass how many grams of water will be produced
Answers
Se producirán 36 gramos de agua
Explicación: Ya que en una reacción química no puede descubrir ningún cambio en masa total de las sustancias que en ella intervienen, pues en una reacción química ni se gana ni se pierde masa.
If 550 grams of KClO3 breaks down and produces 175 grams of KCl, how many grams of O2 are produced?
Answers
Answer:
215.51 g of O2.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
2KClO3 —> 2KCl + 3O2
Next, we shall determine the mass of KClO3 that decomposed and the mass of O2 produced from the balanced equation. This is illustrated below:
Molar mass of KClO3 = 39 + 35.5 + (16×3)
= 39 + 35.5 + 48
= 122.5 g/mol
Mass of KClO3 from the balanced equation = 2 × 122.5 = 245 g
Molar mass of O2 = 16 × 2 = 32 g/mol
Mass of O2 from the balanced equation = 3 × 32 = 96 g
From the balanced equation above,
245 g of KClO3 decomposed to produce 96 g of O2.
Finally, we shall determine the mass of O2 produced by the decomposition of 550 g of KClO3. This can be obtained as follow:
From the balanced equation above,
245 g of KClO3 decomposed to produce 96 g of O2.
Therefore, 550 g of KClO3 will decompose to produce =
(550 × 96)/245 = 215.51 g of O2.
Therefore, 215.51 g of O2 were obtained from the reaction.
Iodine pentafluoride gas reacts with iodine fluoride gas producing iodine heptafluoride gas and iodine gas. What is the maximum number of grams of iodine gas that can be produced from a reaction of 10.0 g of iodine pentafluoride with 11.20 L of iodine fluoride gas at STP
Answers
Answer:
63.45g of I₂ can be produced
Explanation:
IF₅ reacts with IF to produce IF₇ and I₂. The reaction is:
IF₅ + 2 IF → IF₇ + I₂
Moles of 10.0g of IF₅ (221.89g/mol):
10.0g IF₅ × (1mol / 221.89g) = 0.0451 moles of IF₅
Using PV / RT = n, it is possible to find moles of 11.20L of IF, thus:
1atm×11.20L / 0.082atmL/molK × 273K = 0.500 moles of IF.
At STP, pressure is 1atm, temperature is 273K and gas constant R is 0.082atmL/molK
For a complete reaction of IF₅ you need:
0.0451 moles of IF₅ × (2 moles IF / 1 mole IF₅) = 0.902 moles of IF. As you have just 0.500 moles of IF, the IF is the limiting reactant.
2 moles of IF produce 1 mole of I₂. 0.500 moles of IF produce:
0.500mol IF ₓ ( 1 mol I₂ / 2 mol IF) = 0.250 mol I₂
As molar mass of I₂ is 253.81g/mol, mass of 0.250 mol I₂ are:
0.250mol I₂ ₓ (253.81g / mol) =
63.45g of I₂ can be producedlphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Hydrogen’s emission spectrum includes a line of violet light that has a frequency of 7.31 * 10^14 Hz. What is the energy (in joules) a photon of the violet light?
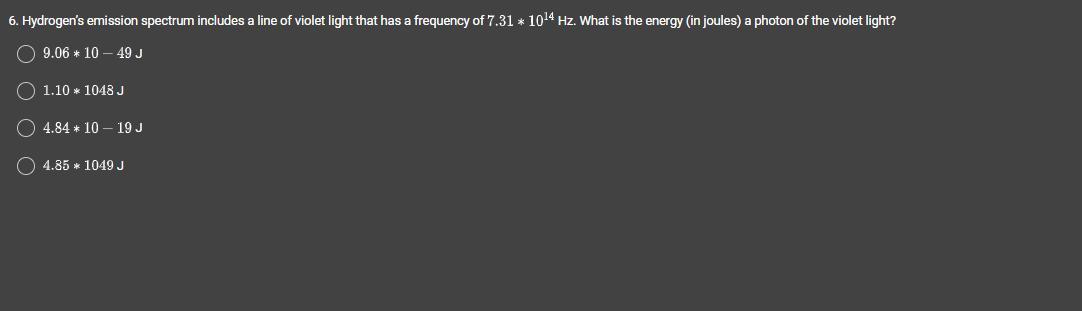
Answers
Explanation:
The energy of a photon of the violet light can be obtained as follows:
\(E\text{ = h x f}\)E = energy
h = Plank's constant
f = frequency
-------
Data provided:
f = 7.31x10^14 Hz
--
Data needed:
h = 6.63x10^-34 Js
So, E = h x f = 6.63x10^-34 Js x 7.31x10^14 Hz = 4.85x10^-19 J
Answer: 4.84x10^-19 J (nearest value)
Oxygen isotope analyses of both deep-sea cores and ice cores (from the Greenland ice sheet) show that the Pleistocene ______.
Answers
Oxygen isotope analyses of both deep-sea cores and ice cores (from the Greenland ice sheet) indicate that the Pleistocene was characterized by frequent and widespread climate variability.
This included cycles of glacial advance and retreat.
These changes were caused by variations in the Earth's orbit and tilt, affecting the amount of solar radiation received by different parts of the Earth.
The Pleistocene also saw a series of major ice ages, during which ice sheets and glaciers covered most of the Earth's surface. These ice ages were interspersed with shorter, warmer periods called interglacials. During that time, the Earth's climate resembled the present climate.
Read more about Pleistocene at:
brainly.com/question/28102167
#SPJ4
Hi! I need some solar system facts!
Answers
Answer: I can help with that!
Explanation: 1.The tremendous growth in the U.S. solar industry is helping to pave the way to a cleaner, more sustainable energy future. Over the past few years, the cost of a solar energy system has dropped significantly -- helping to give more American families and business access to affordable, clean energy.
Through a portfolio of R&D efforts, the Energy Department remains committed to leveraging America’s abundant solar energy resources -- driving research, manufacturing and market solutions to support widespread expansion of the nation’s solar market.
2.The amount of sunlight that strikes the earth's surface in an hour and a half is enough to handle the entire world's energy consumption for a full year. Solar technologies convert sunlight into electrical energy either through photovoltaic (PV) panels or through mirrors that concentrate solar radiation. This energy can be used to generate electricity or be stored in batteries or thermal storage.
Below, you can find resources and information on the basics of solar radiation, photovoltaic and concentrating solar-thermal power technologies, electrical grid systems integration, and the non-hardware aspects (soft costs) of solar energy. You can also learn more about how to go solar and the solar energy industry. In addition, you can dive deeper into solar energy and learn about how the U.S. Department of Energy Solar Energy Technologies Office is driving innovative research and development in these areas.
3.Solar radiation is light – also known as electromagnetic radiation – that is emitted by the sun. While every location on Earth receives some sunlight over a year, the amount of solar radiation that reaches any one spot on the Earth’s surface varies. Solar technologies capture this radiation and turn it into useful forms of energy.
Which of the following best describes the arrangement of particles in a gas?
O The particles are spread apart and can move freely.
O The particles are packed closely together and can move freely.
O The particles are packed closely together and cannot move freely
O The particles are spread apart and cannot move freely.
Answers
Answer: The particles are spread apart and can move freely.
The products of nuclear reaction usually have a different mass than the reactants why?
Answers
Answer:
Explanation:
The best way to explain this is to use an example
\(I\frac{125}{53} + e \frac{0}{-1} ====> Te\frac{125}{52}\)
You have to understand what happened. A electron was shot into the nucleus of the Iodine. That electron change the entire composition of the nucleus resulting in 52 protons. The mass remained the same (125) but the nucleus was altered. The chemical became 125 52 Tellurium. But what is important is that it takes a tremendous amount of energy to disrupt a nucleus, and a new chemical is born from that disruption.
A flask contains 85.5 grams C12H2011 (sucrose) in 1.00 L of solution. What is the molarit
Your answer.
3.8 M
25 M
10M
1.2M
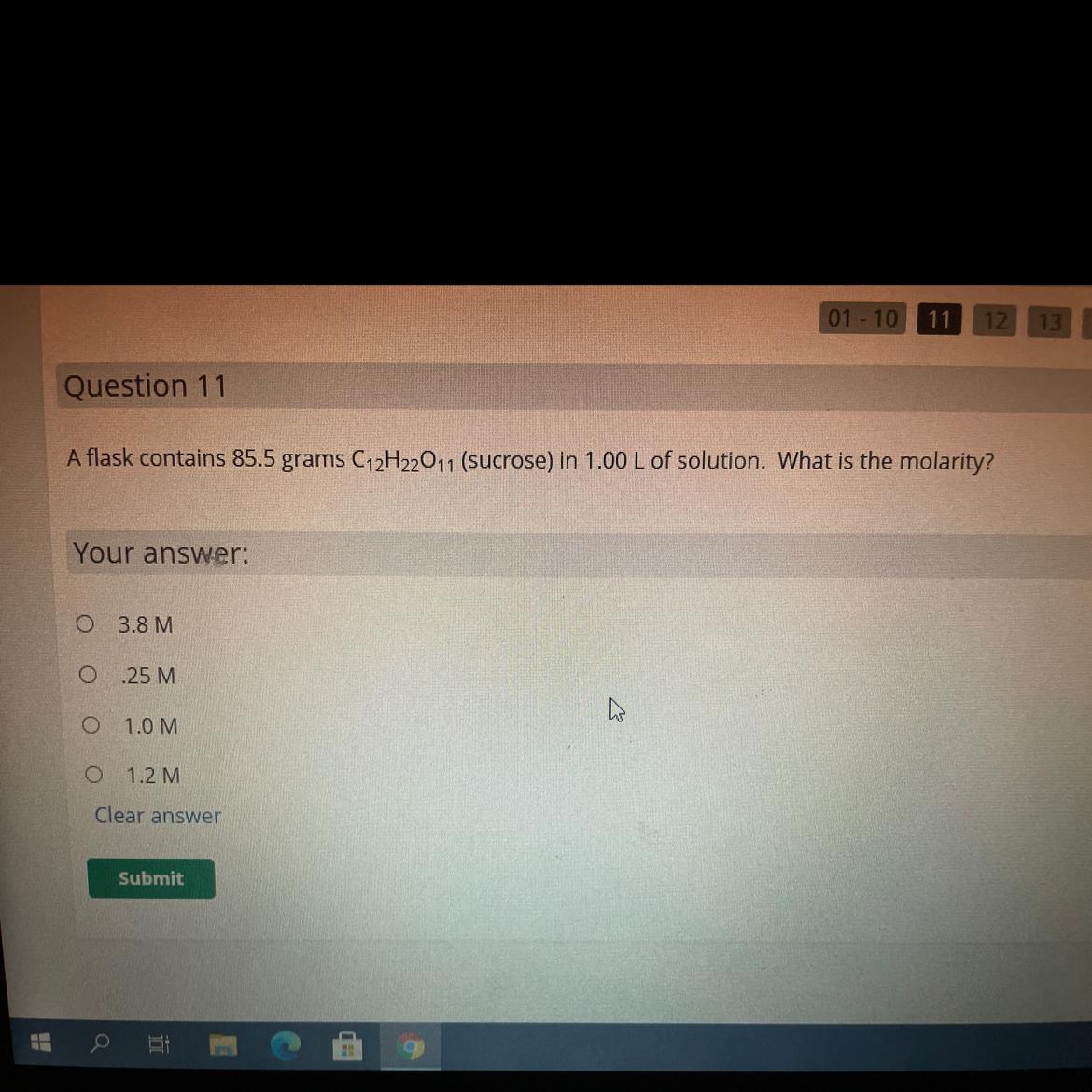
Answers
Answer:
0.25 M
Explanation:
First we convert 85.5 grams of sucrose into moles, using its molar mass:
Molar Mass of C₁₂H₂₂O₁₁ = (Molar Mass of C)*12 + (Molar Mass of H)*22 + (Molar Mass of O)*11Molar Mass of C₁₂H₂₂O₁₁ = 342.3 g/mol85.5 g ÷ 342.3 g/mol = 0.25 molThen we divide the number of moles by the number of liters to calculate the molarity:
0.25 mol / 1.00 L = 0.25 M1. HCI (aq) + NaOH (aq) → NaCl (aq) + H₂O (1)
a. What are the reactants?
b. What are the products?
Answers
In the given reaction, the reactants are hydrochloride acid (HCI) and sodium hydroxide (NaOH). The products are sodium chloride (NaCl) and water.
The chemical equation provided represents a neutralization reaction between hydrochloric acid (HCI) and sodium hydroxide (NaOH) in aqueous solution.
A neutralization reaction is a type of double displacement reaction in which an acid and a base react to form salt and water.
The reactants in this equation are hydrochloric acid (HCI) and sodium hydroxide (NaOH). Hydrochloric acid is a strong acid that dissociates in water to form hydrogen ions (H+) and chloride ions (Cl-). Sodium hydroxide, on the other hand, is a strong base that dissociates in water to form sodium ions (Na+) and hydroxide ions (OH-).
To learn more about reactants, follow the link:
https://brainly.com/question/29816521
#SPJ1
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be true
for heat to transfer from sample A to sample B?
The average kinetic energy of A is greater than that of B.
The average kinetic energy of B is greater than that of A.
The average kinetic energy of both samples is equal.
The average kinetic energy does not determine the direction of heat transfer.
Mark this and return
Save and Exit
56:26
Next
Submit
Answers
How could the age be interpreted in a rock in which the blocking temperature has been reached?
Answers
Which statement is true of energy in reactants during an endothermic reaction?(1 point)
The energy found in the reactants remains in the system, and the reactants also take energy from the surroundings.
All of the energy from the reactants will be lost to the surroundings.
All of the energy from the reactants will remain in the system
Some of the energy in the reactants will remain in them after the reaction, but some is lost to the surroundings. plz anwres right for extra points
Answers
The energy found in the reactants remains in the system, and the reactants also take energy from the surroundings.
A 150-lb patient is prescribed acetaminophen at 7.34 mg/kg. If acetaminophen is available on hand as a 0.125 mg/ml syrup, how many mililiters will the nurse administer?
Answers
Answer:
The nurse should administer 3995.2 millilitres of syrup.
Explanation:
The conversion factor of pound weight to kilogram is; 1 lb = 0.453592 kg
The body weight of the patient in Kg = 150 * 0.453592 = 68.04 kg
A prescription of 7.34 mg/kg acetaminophen means that he should receive;
7.34 mg * 68.04 = 499.4 mg of acetaminophen
Amount in grams of acetaminophen per millilitre of syrup = 0.125 mg
Number of millilitres of syrup required = 499.4 mg / 0.125 mg/mL = 3995.2 mL
Therefore, the nurse should administer 3995.2 millilitres of syrup.
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
Chemistry problems
1. 1.5 moles of potassium sulfate (K SO4) were dissolved in 1000 grams of water (H2O). Find the % and Cm.
2. 10 grams of sulfuric acid (H2SO4) was added to 500 ml of 10% solution of potassium hydroxide (KOH) with a density of 1.1 g/ml. Find the mass of potassium sulfate (K SO4) formed.
3. Find the mass of the salt formed by the reaction of 7.3 grams of hydrochloric acid (HCl) with 5.6 liters (5600 ml) of ammonia (NH3).
4. Find the volume of hydrogen gas (H2) produced by the reaction of 13 grams of zinc with a solution containing 30 grams of sulfuric acid (H2SO4).
5. How much of the concentrated original solution (70%) of acetic acid is needed to prepare 500 grams of 3% (percentage solution)?
Answers
1. The % concentration is 20.7% and the molar concentration, Cm, is 1.5 M.
2. 7.8 grams of potassium sulfate will be formed.
3. 10.7 grams of ammonium chloride will be formed.
4. The volume of hydrogen gas that will be produced is 3.86 liters.
5. 21.43 grams of the 70% acetic acid is needed to prepare 500 grams of 3% acetic acid solution.
What is the percentage concentration?1. Mass of potassium sulfate = 1.5 moles * (174.26 g/mol) = 261.39 g
Mass of water (H₂O) = 1000 g
% = (mass of solute/mass of solution) x 100
% = (261.39 g / (261.39 g + 1000 g)) x 100
% ≈ 20.7%
Cm = moles of solute / volume of solution
Moles of potassium sulfate (K2SO4) = 1.5 moles
Volume of water (H2O) = 1000 g / (density of water) = 1000 g / 1 g/mL = 1000 mL = 1 L
Cm = 1.5 moles / 1 L
Cm = 1.5 M
2. The balanced equation for the reaction is:
H₂SO₄ + 2 KOH → K₂SO₄ + 2 H₂O
Molar mass of sulfuric acid (H₂SO₄) = 98.09 g/mol
Moles of sulfuric acid = 10 g / 98.09 g/mol
Moles of sulfuric acid = 0.102 mol
Based on the mole ratio of the reaction, 0.102 moles of sulfuric acid will react to form 0.102 moles of potassium sulfate.
Molar mass of potassium sulfate = 174.26 g/mol
Mass of potassium sulfate = 0.102 mol x 174.26 g/mol
Mass of potassium sulfate ≈ 17.8 g
3. The balanced equation for the reaction is:
HCl + NH₃ → NH₄ClMolar mass of hydrochloric acid (HCl) = 36.46 g/mol
Moles of hydrochloric acid (HCl) = 7.3 g / 36.46 g/mol
Moles of hydrochloric acid ≈ 0.2 mol
Based on the mole ratio of the reaction, 0.2 moles of hydrochloric acid will react to form 0.2 moles of ammonium chloride.
Molar mass of ammonium chloride (NH₄Cl) = 53.49 g/mol
Mass of ammonium chloride = 0.2 mol x 53.49 g/mol
Mass of ammonium chloride ≈ 10.7 g
4. The balanced equation for the reaction is:
Zn + H₂SO₄ → ZnSO₄ + H₂Molar mass of zinc (Zn) = 65.38 g/mol
Moles of zinc = 13 g / 65.38 g/mol
Moles of zinc ≈ 0.199 mol
Based on the mole ratio of the reaction, 0.199 moles of zinc will react to produce 0.199 moles of hydrogen gas.
Volume of sulfuric acid = 30 g / (density of H₂SO₄ )
The density of H₂SO₄ is 1.84 g/mL
Volume of sulfuric acid = 30 g / 1.84 g/mL
Volume of sulfuric acid ≈ 16.3 mL or 0.0163 L
Using the ideal gas law, the volume of hydrogen gas produced will be:
V = nRT / P
V = (0.199 mol)(0.0821 L·atm/(mol·K))(273 K) / (1 atm)
V ≈ 3.86 L
5. Assuming that the concentrated original solution of acetic acid is 100% acetic acid (CH₃COOH).
Mass of acetic acid = 500 g x (3/100) = 15 g
The concentrated original solution, however, is 70% acetic acid.
70% acetic acid (mass) = 100% acetic acid (unknown mass)
0.7 * (unknown mass) = 15 g
Solving for the unknown mass:
unknown mass = 15 g / 0.7
unknown mass ≈ 21.43 g
Learn more about percentage concentration at: https://brainly.com/question/18761928
#SPJ1
A dramatic classroom demonstration involves cooling a balloon from room temperature (293 K ) to liquid nitrogen temperature (77 K). If the initial volume of the balloon is 2.6 L , what will its volume be after it cools
Answers
The volume of the balloon after it cools is 0.68 L.
Charles law states that the volume of a gas is directly proportional to its temperature at constant pressure.
It is given by:
V ∝ T
V/T = constant
Therefore:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2} \\\\V_1=2.6\ L,T_1=293\ K,T_2=77\ K:\\\\\frac{2.6}{293}=\frac{V_2}{77} \\\\V_2=\frac{2.6}{293}*77\\\\V_2=0.68\ L\)
Hence the volume of the balloon after it cools is 0.68 L.
Find out more at: https://brainly.com/question/16927784
A gas takes up a volume of 25 liters, has a pressure of 2.3 atm, and a temperature of 299 K.
If I raise the temperature to 325 K and lower the pressure to 1.2 atm, what is the new volume of the gas?
Answers
The new volume of the gas is 56.6 liters when the temperature is raised to 325 K and the pressure is lowered to 1.2 atm.
PV = nRT
Where R is the ideal gas constant. Since the number of moles is constant in this problem, we can simplify the ideal gas law to:
P1V1/T1 = P2V2/T2
Where the subscripts 1 and 2 refer to the initial and final states of the gas, respectively.
We can now plug in the given values for the initial state of the gas:
P1 = 2.3 atm
V1 = 25 L
T1 = 299 K
And the given values for the final state of the gas:
P2 = 1.2 atm
T2 = 325 K
We can then solve for V2:
P1V1/T1 = P2V2/T2
(2.3 atm)(25 L)/(299 K) = (1.2 atm)V2/(325 K)
V2 = (2.3 atm)(25 L)(325 K)/(1.2 atm)(299 K)
V2 = 56.6 L (rounded to three significant figures)
for more question on gas
https://brainly.com/question/26758935
#SPJ11
In which region is there most likely to be a volcano? A.D B.B C.A D.C

Answers
Answer: A and D
Explanation:
I hope this helps. Sorry if I’m wrong
Answer:
Region D
Explanation:
The region is on the tectonic plate boundaries.
