removes nitrogen from the atmosphere. removes nitrogen from the atmosphere. denitrification nitrification mineralization nitrogen fixation
Answers
Removes nitrogen from the atmosphere is nitrogen fixation .
What is meant by nitrogen fixation?
In order to create additional nitrogen molecules (including ammonia, nitrate, and nitrogen dioxide) that are helpful for other chemical processes, nitrogen must first be converted from its stable gas form (N) in the air. This process is known as nitrogen fixation. As part of the nitrogen cycle, it is important.
One of the most significant biochemical processes on Earth is nitrogen fixation since it is the main source of the nitrogen in the soil that plants require to develop .Rhizobium is an illustration of a symbiotic nitrogen-fixing bacterium.
Chemically, nitrogen fixation transforms atmospheric nitrogen into ammonia, which is ingested by living things. In essence, nitrogen fixation transforms atmospheric nitrogen into a form that plants can use more easily.
To learn more about nitrogen fixation refer to:
https://brainly.com/question/13485053
#SPJ4
Related Questions
what type of chemical bond forms between two atoms bearing opposite charges?
Answers
Ionic bonds are created when two oppositely charged ions are attracted to one another by electrostatic force. A chemical element is uniquely defined by its atoms, which are tiny pieces of substance.
An atom is made up of a core nucleus and one or more negatively charged electrons that orbit it. The positively charged, comparatively hefty protons and neutrons that make up the nucleus may be present. Any elementary particle of matter with at least one proton is referred to as an atom. Examples of atoms are neon (N) and hydrogen (H) (Ne). Chemistry's fundamental building component is an atom. It is the lowest fraction of substance into which electrically charged particles cannot be released. It is also the smallest piece of substance with chemical element-like characteristics.
learn more about atom here:
https://brainly.com/question/13654549
#SPJ4
An asteroid in space has traveled 4,500 km in 60 s, what is the average speed of the asteroid?
Answers
Which description BEST describes a particle that contains 18 electrons, 18 neutrons and 16 protons?
A) an ion with a charge of negative 2
B) an ion with a charge of positive 2
C) an atom with an atomic number of 18
D) an atom with a mass of 34 amu
Answers
Answer:
C
Explanation:
C) an atom with an atomic number of 18
_____Is a non renewable fuel created from the remains of living organisms.
Electricity
Biofuel
Fossil fuel
Nuclear energy
Answers
q=The+diagram+below+represents+the+building+block+of+a+DNA+molecule+which+is+a+type&rlz=1CATPIA_enUS942&sxsrf=ALeKk02q0TrtMmY64OTcRfnI6UtHtyfVwg:1614616724451&source=lnms&tbm=isch&sa=X&ved=2ahUKEwi2l7yCxI_vAhVjg-AKHXaSCVcQ_AUoAXoECBIQAw&biw=1517&bih=726#imgrc=YTAoiVTgM8UvnM
Answers
Answer:
we need to see the diagram
Explanation:
A 3.458 g sample of KHP, a monoprotic acid, requires 45.71 mL of a KOH solution to reach the endpoint. What is the concentration of the KOH solution? The molar mass of KHP is 204.22 g/mol. x 10! M KOH Enter your answer in scientific notation.
Answers
The concentration of the KOH solution is 0.100 M. To calculate the concentration of the KOH solution, we can use the formula:
Molarity (M) = (moles of solute) / (volume of solution in liters)
Given:
Mass of KHP (potassium hydrogen phthalate) = 3.458 g
Molar mass of KHP = 204.22 g/mol
Volume of KOH solution = 45.71 mL = 0.04571 L
First, we need to calculate the moles of KHP:
moles of KHP = (mass of KHP) / (molar mass of KHP)
moles of KHP = 3.458 g / 204.22 g/mol ≈ 0.01693 mol
Next, we can calculate the concentration of the KOH solution:
Molarity of KOH solution = (moles of KOH) / (volume of solution)
Molarity of KOH solution = 0.01693 mol / 0.04571 L ≈ 0.370 M
Converting the concentration to scientific notation, we have:
Molarity of KOH solution ≈ 3.70 x 10^-1 M
Therefore, the concentration of the KOH solution is approximately 0.100 M.
Learn more about molarity here: brainly.com/question/31545539
#SPJ11
What is a total eclipse?
A) When the Sun partially blocks out
the moon.
B) When the moon partially blocks
out the Sun.
C) When the moon blocks out the
Sun entirely.
D) When the Sun blocks out the
moon entirely.
Answers
Hope it helps ~
:-:> patulong po <:-:
TRUE or FALSE
1. Gravity is a contact force that acts between two seperate objects.
2. Gravity is the force exerted by the Earth which is equivalent to the mass of the object.
3. Gravity on the Moon is less than the gravity on the Earth.
4. Friction, unlike gravity, is a non-contact force.
5. Friction keeps objects from sliding off surfaces.
6. The principle of gravity was discovered by Sir Isaac Newton.
7. Friction is the force that opposes the push of gravity on objects.
8. The lesser the mass of an object, the greater the pull of gravity on it.
9. Gravity keeps anything from going up.
10. Gravitational pull between objects increases as the distance between them decreases.
Answers
Answer:
1. FALSE
2. FALSE
3. TRUE
4. FALSE
5. TRUE
6. TRUE
7. FALSE
8. FALSE
9. FALSE
10. FALSE
How many moles of H2O are in 359 grams of H2O
Answers
Answer:
359
i think
Explanation:
Consider a two step synthetic sequence to generate the chiral cyclopropane. provide the alkyne starting material a, intermediate organic product b, and identify the reagents.
Answers
The formed cyclopropane has cis stereochemistry.
Chiral cyclopropane rings are important pharmacophores in pharmaceutical drugs and bioactive natural products, so libraries of these building blocks are an important resource for drug research and development campaigns.
A synthesis is a set of two or even more reactions that are designed to produce a specific end product. A synthetic step is a single reaction that has to be carried out independently of the other reactions in a synthesis.
The number of steps inside a synthetic sequence equals the number of reactions that need to be performed separately, i.e. the number of reactions that comprise the sequence.
By stereospecific addi-tion of singlet carbenes, cis cyclopropane could be synthesized from cis alkene. Using a Lindlar catalyst, cis alkene could be synthesized from alkynes.
To know more about the cyclopropane, here
https://brainly.com/question/29385628
#SPJ4

M. element
cenabrecht
0.5 mele of an
Answers
Answer:
Number of moles (n)=
molecular weight
weight
Weight=n×Molecular weight
=0.5×14
Mass=7g
13.0 mol 3.0 mol methanegasand oxygengas react to form carbon dioxide gas and watervapor. suppose you have of and of in a reactor. suppose as much as possible of the reacts. how much will be left? round your answer to the nearest
Answers
The no. of moles of \(CH_4\) remaining after the reaction is found to be 11.5 moles.
The no. of moles of a material equals the ratio of its given mass to the mass of one mole of that molecule in a chemical reaction.
The coefficients reflect the number of moles, not simply molecules, that react.
Stoichiometric coefficients are the numbers that come before the chemical formula of a molecule participating in a reaction. They assist us in determining the relative amounts or no. of moles of each reactant consumed and product created during a reaction.
\(CH_4 (g) + 2O_2 (g) \rightarrow CO_2 (g) + 2H_2O (g)\)
No. of moles of \(CH_4\) = 13.0 mol
No. of moles of \(O_2\) = 3.0 mol
Here \(O_2\) is limiting reagent because moles of \(O_2\) is less.
Thus, \(CH_4\) is in excess
1 mol \(CH_4\) = 2 mol \(O_2\)
x mol \(CH_4\) = 3 mol \(O_2\)
Moles of \(CH_4\) used = 3 x 1 / 2 = 1.5 mol
Here we have 13 moles of \(CH_4\),
Therefore,
No. of moles of \(CH_4\) left = 13 - 1.5 = 11.5
Result:
11.5 moles of \(CH_4\) will remain after the reaction.
Learn more about No. of moles used in a reaction here:
https://brainly.com/question/15364329
#SPJ4
Out going shortwave radiation depends on:
Answers
Outgoing shortwave radiation depends on factors such as the angle of the Sun's rays, albedo, cloud cover, and atmospheric gases. These factors collectively determine the amount of solar radiation that is reflected, absorbed, and re-emitted by the Earth's surface, influencing the outgoing shortwave radiation.
Outgoing shortwave radiation depends on several factors. Firstly, it is influenced by the solar radiation received by the Earth's surface. The amount of solar radiation reaching the Earth is determined by the angle of the Sun's rays, which changes throughout the day and across different seasons. This means that outgoing shortwave radiation will vary depending on the time of day and the time of year.
Another important factor is the albedo, which refers to the reflectivity of different surfaces on Earth. Surfaces with high albedo, such as ice and snow, reflect more solar radiation back into space, resulting in lower outgoing shortwave radiation. Conversely, surfaces with low albedo, such as dark soil and vegetation, absorb more solar radiation, leading to higher outgoing shortwave radiation.
The presence of clouds also plays a role in outgoing shortwave radiation. Clouds can either reflect incoming solar radiation back into space or absorb and re-emit it as longwave radiation. The type and thickness of clouds, as well as their altitude, can affect the amount of outgoing shortwave radiation.
Finally, atmospheric gases such as water vapor, carbon dioxide, and ozone can also influence outgoing shortwave radiation. These gases absorb and re-emit some of the incoming solar radiation, impacting the amount of radiation that escapes back into space.
Learn more about shortwave here :-
https://brainly.com/question/31450158
#SPJ11
Calculate the molality of chloride ions in an aqueous solution of magnesium chloride in which the mole fraction of magnesium chloride is 0.0400 .
Answers
The molality of chloride ions is 4.63kg/L
Data;
Mole Fraction = 0.0400Molality of Chloride ions = ?Molality of Chloride ionsMole Fraction of MgCl2 = 0.04
The sum of mole fraction = 1
Mole fraction of water = 1 - 0.04 = 0.96
since the total number of moles = 1
Number of moles of MgCl2 = mole fraction * total number of moles
Number of moles of MgCl2
\(0.04 * 1 = 0.04 moles\)
Number of moles of water =
\(0.96 * 1 = 0.96 moles\)
1 moles of MgCl2 = 2 moles of Chloride ions
Number of moles of chloride ions
\(2 * 0.04 = 0.08 moles\)
Number of moles of water = 0.96moles
mass of water = molecular weight * number of moles
mass of water =
\(18 * 0.96 = 17.28g\)
let's convert this to kilogram
\(1000g = 1kg\\17.28g = x\\x = 0.01728kg\\\)
The molality of the ions in the solution is
\(M = \frac{mass of solute}{volume of solution} \\M = \frac{0.08}{0.01728} =4.63 kg/L\)
The molality of chloride ions is 4.63kg/L
Learn more on molality of ions here;
https://brainly.com/question/10278982
Barium ions carry a 2 charge, and nitrogen ions carry a 3- charge. what would be the chemical formula for the ionic compound barium nitride?
a. ba2n3
b. ba3n2
c. ba2n2
d. ba3n4
Answers
The correct chemical formula for barium nitride is: b. Ba3N2
The chemical formula for barium nitride can be determined by balancing the charges of the ions involved. Barium ions carry a 2+ charge, while nitrogen ions carry a 3- charge.
To balance the charges, we need two barium ions (2 × 2+ = 4+) for every three nitrogen ions (3 × 3- = 9-). The positive and negative charges must cancel each other out in the compound.
Therefore, the correct chemical formula for barium nitride is: b. Ba3N2
Learn more about chemical formula https://brainly.com/question/11574373
#SPJ11
Match up the characteristics below with the type of molecular bond they describe. Bonds found in Halite (between Na+ and Cl-) Bonds found between Si and O in the Si-O tetrahedron Bonds inside the water molecule (between the H and O ) Bonds that exist between two water molecules Strongest bond type Weakest bond type Bonds that are used by water to dissolve sal
Answers
The characteristics and the type of molecular bond they describe:
1. Bonds found in Halite (between Na⁺ and Cl⁻): Ionic bond
2. Bonds found between Si and O in the Si-O tetrahedron: Covalent bond
3. Bonds inside the water molecule (between the H and O): Covalent bond
4. Bonds that exist between two water molecules: Hydrogen bond
5. Strongest bond type: Covalent bond
6. Weakest bond type: Van der Waals bond
7. Bonds that are used by water to dissolve salt: Ionic bond
The ionic bond is a type of molecular bond found in halite (between Na⁺ and Cl⁻). The Si-O tetrahedron is held together by a covalent bond. The bond inside the water molecule (between the H and O) is also a covalent bond. The hydrogen bond is the type of bond that exists between two water molecules. The covalent bond is the strongest bond type, while the van der Waals bond is the weakest bond type. Water uses the ionic bond to dissolve the salt.
Learn more about bonds: https://brainly.com/question/32306693
#SPJ11
how many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with of water? mg3n2 3h2o → 2nh3 3mgo question 22 options: 0.114 0.0756 0.429 4.57 0.0378
Answers
The reaction will produce 6.61 mole of magnesium oxide.
What is the purpose of magnesium oxide?
There are several uses for magnesium oxide. Some people use it as an antacid to treat acid indigestion, digestive problems, and heartburn. Magnesium oxide can also be used as a laxative to quickly and temporarily empty the bowels (before surgery, for example).
Briefing:
This clearly demonstrates that 100.95g magnesium nitride reacts with 108.06g of water to give stoichiometric ammonia and magnesium oxide.
moles of Mg3N2 = 3.82g /100.95g per mole =0.0387 mole
mole of OH2 = 7.73g/18.01g per mole
magnesium oxide, i.e. 0.0387 mole × 58.32g per mole= 6.61mole
To know more about magnesium oxide visit:
https://brainly.com/question/1533548
#SPJ4
What type of reaction do Carbon -14 and Uranium- 238 undergo? Explain how you figured this out and write the reaction for each
Answers
The reaction for Carbon-14, used in carbon dating, decays by beta emission and in Uranium-238 decays by alpha emission.
Alpha radiation releases when the nucleus of an atom becomes unstable and alpha particles are released in order to restore stability. Alpha decay occurs in elements have high atomic numbers, such asuranium, radium, and thorium etc. The reaction that describes an alpha emission because radiations are 5740 years. Now, Carbon-14 has a half life of 5730 yrs, and it used to date fossils of 50 hundred yrs old. It undergo beta emission. In case of Uranium- 238, has half life of 236 yrs. Because there is so much difference between half lives of both so we can't use both of together in one reaction. So, it goes on alph emission. The reactions are
¹⁴₆C → ¹⁴₇N - e⁻
Hence, required reaction is alpha emmision.
For more information about alpha emission, visit :
https://brainly.com/question/27875918
#SPJ4
what is the IUPAC name for H2S (the 2 supposed to be down)
Answers
The correct IUPAC name for H2S (with the 2 written as a subscript) is hydrogen sulfide.
Easy ,
Having problems figuring out molar mass in general. steps would be most appreciated.
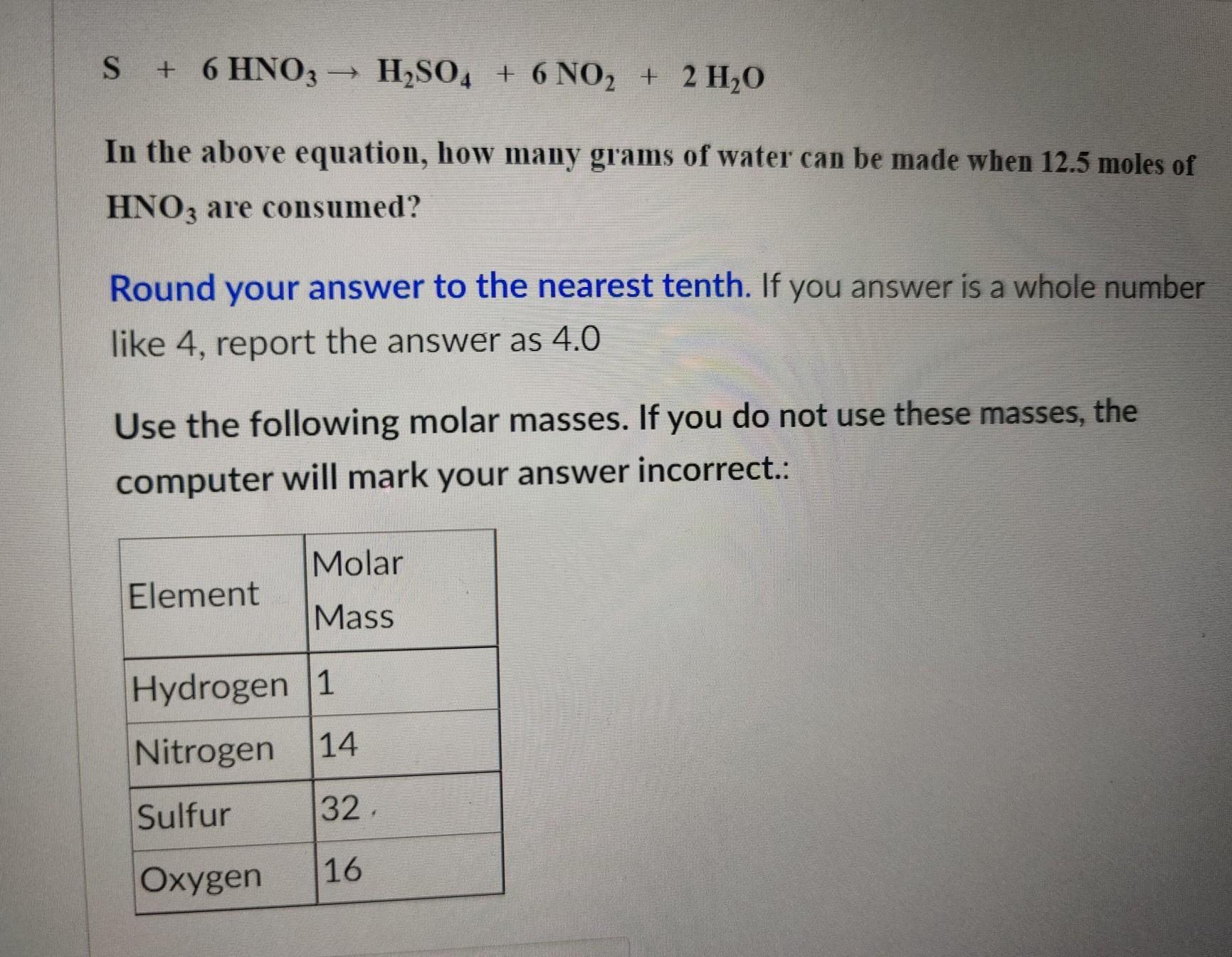
Answers
Answer:
The periodic table
Explanation:
As you can see in the screenshot. Each element on the periodic table has an atomic number and a mass number. The atomic numbers are arranged in numerical order from right to left in the periodic table. The second number there is the atomic mass.
When doing stoichiometric ratios, you need to use the atomic mass of each element to determine the molecular/molar mass.
example. Find the molar mass of
H₂SO₄
Hydrogen (2) + sulphur (1) +oxygen (4)
= atomic mass of H(2)+ atomic mass S(1)+ atomic mass oxygen(4)
=1(2) + 32(1)+16(4)
=2+32+64
=98
N.B We do not use the co-efficient when looking for molar mass, we only use the atom and subscript
Benedict College E360 xG The number of second o x The cis isomer of the 1.3-diethyl cyclobutane is: CHyCH2 CH CH CH CHy CH CHs OA ?? oc Which is more stable,cis-1-ethyl-3-methylcyclohexane or trons-1-ethyl-3-methylcyclohexane? O cis trans The number of second degree (2") hydrogens in the structure below is ViewSonic
Answers
A cyclic diallylamine called 1-ethyl-3-methylcyclohexane is a feedstock used to create methicillin-resistant Staphylococcus aureus (MRSA).
Describe trons-1-ethyl-3-methylcyclohexane or methylcyclohexane.An organic substance with the chemical formula CH3C6H11 is methylcyclohexane. It is a white liquid with a little odor that is categorized as a saturated hydrocarbon.As a solvent, methylcyclohexane is employed. Toluene is the principal product of its conversion in naphtha reformers. [4] Some correction fluids (like White-Out) also use methylcyclohexane as a solvent.Because one methyl group is attached to one carbon of the cyclohexane ring, methylcyclohexane is a monosubstituted cyclohexane.This monosubstituted methylcyclohexane has the lowest energy form when the methyl group is located equatorially as opposed to axially.The idea of A value embodies this equilibrium. Because there are axial hydrogen atoms on the same side of the ring while the methyl group is in the axial position, there is steric crowding (steric strain) experienced by the methyl group (known as the 1,3-diaxial interactions).To know more about methylcyclohexane visit:
https://brainly.com/question/14855251
#SPJ4
What is the geometry of H₂F
Answers
Answer:
ihvjvbvbnbgvdcghffvhh
Consider the following reaction: Solid zinc was added to 1.0 M HC1.
After 20.0 s, the temperature of the container increased by 0.5°C and 25.00 mL of
H2 was produced. The rate of this reaction was
Answers
Answer:
1.25 mL/s
Explanation:
Your are told that 25.0 mL of H2 was produced 20 seconds. This is equal to rate 25/20 = 1.25 mL/s
When a reaction progresses, the reactant concentration gradually decreases and the product concentration gradually increases. Here the rate of the reaction is 1.25 mL/s.
What is rate of reaction?The rate of a chemical reaction is defined as the rate of decrease of concentration of a reactant or the rate of increase of concentration of a product. The rate of the reaction is usually expressed in units mol L⁻¹s⁻¹. The rate of the reaction are affected by several factors like concentration of the reactants, temperature, etc.
An equation which expresses the experimentally observed rate of a reaction in terms of the molar concentration of the reactants which determine the rate of the reaction is called rate equation.
Here it is seen that 25.0 mL of H2 was produced 20 seconds. So the rate is given as:
Rate = 25/20 = 1.25 mL/s
Thus the rate of the reaction is 1.25 mL/s.
To know more about rate of reaction, visit;
https://brainly.com/question/30546888
#SPJ2
2.107 × 10^24 calculate the number of moles
Answers
What is the oxidation number of oxygen in hydrogen peroxide
Answers
Answer:
peroxide such as hydrogen peroxide In peroxide, oxygen has an oxidation number of -1. when oxygen is combined with fluorine it's oxydation number is +2
Label the small containers 1 and 2. Add ¼ cup of room-temperature water to container 1 and ¼ cup of room-temperature vinegar to container 2. Before continuing, make a hypothesis about each combination. Will combining water and baking soda result in a chemical reaction? Will combining vinegar and baking soda result in a chemical reaction?
Answers
When baking soda (NaHCo3) reacts with water it produces a new substance such as carbonic acid which shows it is a chemical reaction because something new is produced. This reaction releases heat energy so we can say that it is an exothermic reaction.
Mixing baking soda and vinegar with each other will produce a new substance such as carbondioxide gas is produced which also show that it is a chemical reaction so we can conclude that both reaction represents chemical reaction.
Learn more: https://brainly.com/question/24906260
Which option does NOT demonstrate a
property of heat?
A. A physical substance.
B. The KE of molecules.
C. A form of energy transfer.
D. It is a form of energy. helllllllllppppppp
Answers
The option that does not demonstrate a property of heat is that it is a physical substance (option A).
What is heat?Heat is the transfer of kinetic energy from one medium or object to another, or from an energy source to a medium or object.
Heat can also refer to the thermal energy transferred between two systems at different temperatures that come in contact.
Heat is a form of energy and not a physical substance. Therefore, the first option is the correct answer.
Learn more about heat at: https://brainly.com/question/25384702
#SPJ1
Explain in your own words, why can’t we predict earthquakes?
Answers
Research has shown, that shaking of an earthquake displays a characteristic pattern. After the first tremors start, building up in intensity, a peak is reached, followed by a fading shaking. Large and small earthquakes start the same way, but there is no way to say when the peak, the maximal magnitude of the quake, is reached.
how many electrons can vanadium lend or borrow
Answers
According to the electronic configuration, vanadium can lend three electrons.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29157546
#SPJ1
It requires energy to melt ice. Applying salt to the ice created a chemical reaction melting the ice. Were did the energy come from to melt the ice? *
Answers
Answer:
HOPE IT HELPS...
Explanation:
When added to ice, salt first dissolves in the film of liquid water that is always present on the surface, thereby lowering its freezing point below the ices temperature. Ice in contact with salty water therefore melts, creating more liquid water, which dissolves more salt, thereby causing more ice to melt, and so on