Answers
Answer:
A.) the molecules that make up the cell membrane
Explanation:
Phospholipids are the molecules that makes up the cell membrane. They are lipid layers which gives support and regulates the movement of materials in and out of the cell.
The phospholipid is very important for the building of membrane round a cell. They are made up of rich lipid layer which is made up of phosphoric acids, nitrogen base, alcohol and fatty acids.
With these molecules, they are able to stabilize and regulate the movement of materials in and outside of the cell.
Related Questions
Balance each of the following equations using the tally method.
*I have provided the answers to this question using the blanks, please show your work using the tally method to earn credit!*
__3___ CrF2 + _____ Al2(SO4)3 → ___2__ AlF3 + ___3__ Cr(SO4)
__2___ Al + __6___ H2O → __2___ Al(OH)3 + __3___ H2
__3___ Ag2(SO4) + __2___ Ga → _____Ga2(SO4)3 + __6___Ag
Answers
A chemical equation is balanced when the number of atoms of each element is the same on both sides of the equation.
Here is the process of balancing a chemical equationStep 1:
Write the unbalanced equation using the correct chemical formulas with reactant on the left-hand-side and product on the right-hand-side.
Step 2:
Count the number of atoms of each element on both sides of the equation. This is done by simply writing the chemical formula for each molecule and multiplying the subscripts by the coefficient.
Step 3:
In case the number of atoms of each element is not equal on both sides, choose an element and adjust the coefficients to balance the number of atoms of that element on both sides.
Step 4:
Repeat step 3 for each element that is not balanced until the equation is balanced.
Step 5:
Check that the number of atoms of each element is the same on both sides and they should be balanced.
Please not that the coefficients are to be in simplest whole number ratio.
Learn more about balancing equation here:
https://brainly.com/question/26694427
#SPJ1
The following reaction take place in a container where CONDITIONS ARE NOT STP! Calculate the volume nitogen dioxide that will be produced when 4,86 dm3 N2O5 decompose. 2N2O5(g) → 4NO2(g) + O2(g)
Answers
9.77 litres of NO2 are generated on average.
Calculation-The balanced equation for the breakdown of N2O5 is as follows:
\(2N_2O_5(g) -- > 4NO_2(g) + O_2(g)\)
determine how many moles of N2O5 decompose:
\(V(N_2O_5) / Vm = n(N_2O_5)(N_2O_5)\)
where V(N2O5) = 4.86 dm3 is N2O5's volume and Vm(N2O5) is N2O5's molar volume under the circumstances stated in the ideal gas law:
\((R*T)/P = Vm = V/n\)
when the gas constant R is used.
the kelvin scale of temperature, T
The pressure is P.
The ideal gas law:
\(n(N_2O_5) = V(N2O5) / Vm(N_2O_5) = 4.86 dm3 / (24.46 L/mol) = 0.1982 mol\)
the number of moles of NO2 is:
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
then,
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
to know more about the reaction here:
brainly.com/question/28984750
#SPJ1
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
653.12 + 12.10247 = [?
Answers
Answer:
665.22
Explanation:
you see wich numbers after the decimal points have the least numbers, the one with the least u have to round your answer to that
After performing the required mathematical operation (addition), the answer is equal to 665.22.
What are significant figures?In Mathematics, significant figures can be defined as the number of single digits or numerical values in the coefficient of a mathematical expression that are important and meaningful.
Since "653.12" has two (2) significant figures, we must ensure that 12.10247 also has two (2) significant figures as follows:
12.10247 to 2 S.F = 12.10.
Next, we would perform the required mathematical operation (addition):
653.12 + 12.10 = 665.22.
Read more on significant figures here: https://brainly.com/question/24491627
#SPJ9
How do the particles in plasmas compare with
the particles in solids?
O Plasmas and solids are both made up of cation-anion pairs.
• Solids and plasmas are both made up of electrons and cations.
Solids are made up of cation-anion pairs, but plasmas are not.
O Plasmas are made up of cation-anion pairs, but solids are not.
Answers
Answer:
Solids are made up of cation-anion pairs, but plasmas are not
Explanation:
Solid is made from cautions and anions while the plasma is not and hence both are made from the cautions and anion plasma. Solids and plasma is made from electrons and solids are made from caution and anion pairs. Plasma is a good conductor of electricity as they have a lot of mobile charged particles.The chemical equation below is unbalanced. CaS + AlC → A + CaC Balance this equation.
Answers
The balanced chemical equation is CaS + AlC → A + CaC
To balance the chemical equation CaS + AlC → A + CaC, we need to ensure that the same number of atoms of each element is present on both sides of the equation. Here's the step-by-step process to balance the equation:
Begin by counting the number of atoms of each element on both sides of the equation.
Left side (reactants):
Calcium (Ca): 1
Sulfur (S): 1
Aluminum (Al): 1
Carbon (C): 1
Right side (products):
A: 1
Calcium (Ca): 1
Carbon (C): 1
Sulfur (S): 0
Start by balancing the elements that appear in the fewest compounds. In this case, we can balance sulfur (S) first. Since there is only one sulfur atom on the left side and none on the right side, we need to add a coefficient of 1 in front of A on the right side to balance the sulfur.
CaS + AlC → 1A + CaC
Next, balance calcium (Ca) by adding a coefficient of 1 in front of CaS on the left side.
1CaS + AlC → 1A + CaC
Now, balance aluminum (Al) by adding a coefficient of 1 in front of AlC on the left side.
1CaS + 1AlC → 1A + CaC
Finally, balance carbon (C) by adding a coefficient of 1 in front of CaC on the right side.
1CaS + 1AlC → 1A + 1CaC
The balanced chemical equation is:
CaS + AlC → A + CaC
For more question on balanced chemical equation visit:
https://brainly.com/question/30196693
#SPJ8
what are examples of health science careers? Select five options

Answers
Answer:
The Health Science Career Cluster has five Health Science pathways:
Support Services.
Therapeutic Services.
Biotechnology Research and Development.
Diagnostic Services.
Health Informatics.
Explanation:
Hi
Can someone explain the second marking point?
Why is it bubbles of air and not C02?



Answers
True or false
Bases will have a relatively high concentration of hydroxide ions and a pH around 10.
Answers
Answer:
false
Explanation:
It is because the pH for Hydroxide ions is 7
Answer:
True
Explanation:
Acids have a low concentration of hydroxide ions and a pH of 1-6, while bases have a high concentration of hydroxide ions and a pH of 8-14
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
consider the equilibrium system described by the chemical reaction below which has a value of kc equal to 1.2 x 10^-4 at a certain temperature. if a solid sample of nh4sh decomposes, what will he equilibrium constant
Answers
If a solid sample of NH₄SH decomposes with an equilibrium constant of 1.2 x 10⁻⁴, the equilibrium concentration of NH₃ will be 1.1 × 10⁻² M.
What is the equilibrium constant?The equilibrium constant (Kc) is the ratio of the concentration of products to the concentration of the reactants, each raised to their respective stoichiometric coefficients. It does not include solids or pure liquids.
Step 1: Write the balanced equation.NH₄SH(s) ⇄ NH₃(g) + H₂S(g)
Step 2: Make an ICE chart.NH₄SH(s) ⇄ NH₃(g) + H₂S(g)
I 0 0
C +x +x
E x x
Step 3: Write the expression of Kc and solve for x.Kc = [NH₃] [H₂S] = x² = 1.2 x 10⁻⁴
x = 1.1 × 10⁻² M
If a solid sample of NH₄SH decomposes with an equilibrium constant of 1.2 x 10⁻⁴, the equilibrium concentration of NH₃ will be 1.1 × 10⁻² M.
The question was incomplete. This is the complete question.
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 10⁻⁴ at a certain temperature. If a solid sample of NH₄SH decomposes, what will the equilibrium concentration of NH₃ be? NH₄SH(s) ⇄ NH₃(g) + H₂S(g)
Learn more about the equilibrium constant here: https://brainly.com/question/19340344
Formulate your hypothesis for the problem. Design an experiment to test your hypothesis.
Answers
making up an answer:
I hypothesize that water will evaporate quicker under an incubator than on the counter.
The experiment; Equal amounts of water in the same type of containers, one under an incubator, and one on a counter with even temperature.
An clement X has 2 electrons in K shell, 8 electrons in L shell and 5 electrons in i Size of X ion is greater than that of X atom though both contain the same protons. Give reason. ii) Write down the formula of one of the compounds of X where X is in -3 oxidation.
Answers
Answer:
i) The size of X ion is greater than that of X atom even though both contain the same number of protons because the ion has fewer electrons compared to the atom. When an atom forms an anion (negative ion), it gains electrons, which causes increased electron-electron repulsion. This repulsion causes the electron cloud to expand, and as a result, the ion becomes larger than the neutral atom.
In the case of element X, when it forms an ion with a -3 charge, it will gain 3 more electrons, increasing the total number of electrons to 18. This will cause the size of the X ion to be larger than the neutral X atom.
ii) To determine the compound of X in the -3 oxidation state, we first need to determine the element's identity. We know that X has 15 electrons in total (2 in the K shell, 8 in the L shell, and 5 in the M shell). Therefore, X has an atomic number of 15, which corresponds to phosphorus (P).
Since phosphorus is in the -3 oxidation state, it gains 3 electrons and becomes P^3-. To form a compound, we need a cation that can balance the negative charge. A common example is aluminum (Al), which has a +3 charge (Al^3+). When phosphorus and aluminum combine, they form the compound aluminum phosphide with the formula AlP.
How many oxygen atoms are represented by the formula Fe(CIO4)3? iron(III) chlorate
3
16
12
4
Answers
Answer:
12
Explanation:
the 4 by the element symbol O multiplied by the 3 on the outside of the parentheses
Answer:
\(\boxed {\boxed {\sf C. \ 12 \ oxygen \ atoms }}\)
Explanation:
We are given the chemical formula:
\(Fe(ClO_4)_3\)
There are 3 elements here:
Fe: Iron Cl: ChlorineO: OxygenThe question asks for the number of oxygen atoms, so we can just focus on the O in the formula.
The O has a subscript of 4, indicating there are 4 oxygen atoms in the compound. But the compound is also enclosed in parentheses with a subscript of 3. Therefore, there are 3 of the compounds with 4 oxygen atoms.
We can multiply 3 and 4.
3*4= 12There are 12 oxygen atoms.
TOPIC: Chemical Reactions
Select the two (2) chemical reactions that are balanced.
OC2 H4 (g) + O2 (g)
OC2 H4 (g) + 302 (g)
Mg(s) + HCL(g)
Mg(s) + 2HCL(g)
CO2 (g) + H₂ O(1)
-> 2CO2 (g) + 2H₂O (1)
-> MgCl2(aq) + H2(g)
-> MgCl2(aq) + H2(g)

Answers
Answer:
C2H4(g) + 3O2(g) ----> 2CO2 + 2H2O(l)
Mg(s) + 2HCL(l) ----> MgCl2(aq) + H2(g)
8. Match the following:
1.Warp
2. Retting
3.Ginning
4.Weft
a. Removal of gunny matter from the stem of a flax or jute plant by bacterial action in stagnant water.
b. The length wise yarn in the loom.
c. The cross wise yarn in the loom. d.. Removal of seeds from cotton
Answers
Answer:
c
Explanation:
Help please from science

Answers
The correct answers to the fill in the blank problems given above are as follows:
Mitotic phase is the part of the cell cycle that divides the material
This process happens only in somatic cells, also is known as body cells.
There are 4 parts of this process:
1. Prophase: the phase that DNA coils into the chromosome.2. Metaphase: the phase where the chromosome organize in the middle of the cell to prepare to divide.3. Anaphase: the phase where chromosome pull apart4. Telophase: the phase where 2 nuclear membranes surround the divided genetic materials What is meant by mitosis?Mitosis can simply be defined as a special process of cell division in which a single cell divides into two different identical daughter cells.
Aside, mitosis; another major cell division which occurs in the cells of living organisms is the meiosis. Below are the phases involved in mitotic cell division, these are as follows:
ProphaseMetaphaseAnaphaseTelophaseIn conclusion, it can be deduced from the explanation given above that mitosis is a process of cell division.
Read more on cell division:
https://brainly.com/question/25578766
#SPJ1
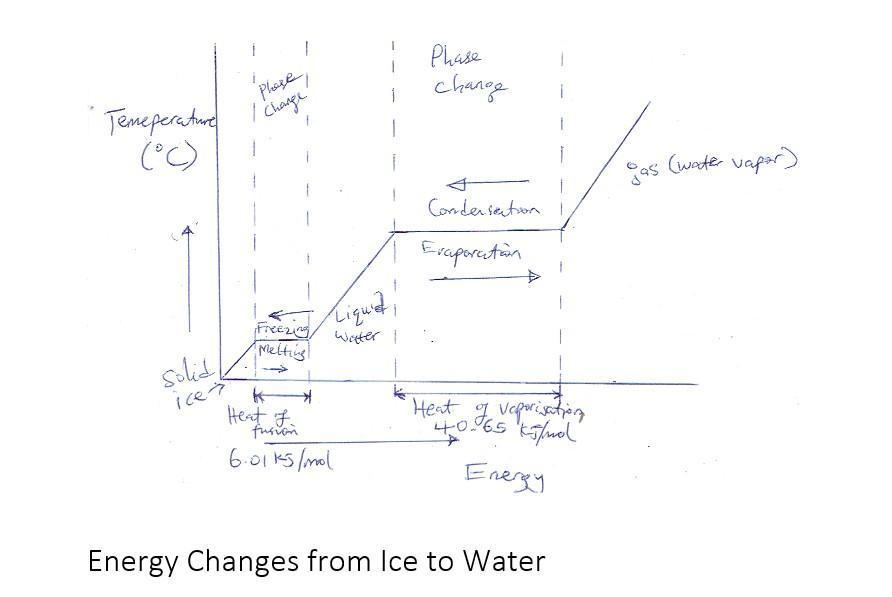
2a. Prepare a schematic diagram of energy changes from ice to water
Answers
Answer:
The change in phases from solid ice to liquid water involves a large amount of energy which is about 334 kJ/kg or 6.01 kJ/mol while the temperature during the phase change remains constant
The constant energy or heat required to realize the change in phase from liquid then to gas is known as the latent heat of fusion which is indicated by plateau in the attached temperature to time graph taken at atmospheric pressure.
Between the plateaus are transitional stages where the water temperature in a particular phase (ice, water or steam) rises
Explanation:
Which of the following is an example of a process that absorbs energy?
gas converting to a liquid
solid coverting to a liquid
a substance freezing
temperature of a substance decreasing
Answers
Answer:
-Gas converting to a liquid
-Solid converting to a liquid
Explanation:
Endothermic process. It's absorb heat
The diagram below shows the branching tree diagram for humans. The text box below it shows the set of derived shared characteristics for the branching tree. A slanting, horizontal line is shown. On the extreme left, there is a label that says Common Ancestor. Along the slanting, horizontal line there are five dots labeled from left to right as 1, 2, 3, 4, and 5. There is one vertical line between each of the consecutive five dots. The lines are labeled from left to right as Perch, Frog, Pigeon, Rats, and Human. A text box below the branching tree diagram is labeled Derived Shared Characteristics. In the box it says from left to right, Terrestrial during all stages, Jaws, Walking on two legs, Mammary glands and hair, and Four limbs. Look at the possible derived shared characteristics, shown in the text box. Think about where these should be placed along the branching tree diagram. From the text box, select a shared derived characteristic that frogs and pigeons have. Explain why you think frogs and pigeons share this characteristic.
Answers
Based on the information provided, the shared derived characteristic that frogs and pigeons have is "Jaws."
Frogs and pigeons both belong to the vertebrate group and possess jaws. Jaws are bony structures that are essential for feeding and play a crucial role in the process of digestion. Frogs have well-developed jaws that allow them to catch and consume prey, while pigeons have a beak that serves as their modified jaw structure.
The branching tree diagram indicates that frogs and pigeons branch off at different points, suggesting that they have evolved independently from a common ancestor.
Learn more about branching tree diagrams, here:
https://brainly.com/question/30876426
#SPJ1
From a laboratory process designed to separate water into hydrogen and oxygen gas, a student collected 20.0g of Hydrogen and 158.6 g oxygen. How much water was originally involved in the process?
Answers
A total of 158.6gram of water were initially used in the process. Through an electrochemical process, it can be divided into hydrogen and oxygen.
How does electrochemistry work?The area of physical chemistry known as electrochemistry studies the relationship between electrical potential difference—a measurable and quantitative phenomenon—and distinguishable chemical change, either as the result of a specific chemical change leading to a potential difference or the other way around.
Given,
Amount of Hydrogen : 20.0 g
Amount of Oxygen : 158.6g
Amount of Water used ?
Therefore,
Mass of Reactant = Mass of product
Mass of Reactant = Mass of Hydrogen + Mass of Oxygen
Mass of Reactant = 158.6 gram + 20 gram
Mass of Reactant (Water) = 178.6 gram
Consequently, 178.6 grams of water were initially used in the process. Hydrogen and oxygen can be separated from it using an electrochemical process.
To know more about electrochemistry visit:
https://brainly.com/question/25025146
#SPJ1
Calculate the PE of an object that has a mass of 2kg that is resting on a 6m table. HELP ME PLEASE I REALLY NEEEEED HELP!!!! 21 POINTS PLEASSEEEE
Answers
Answer:
Calculate the unknown variable in the equation for gravitational potential energy, where potential energy is equal to mass multiplied by gravity and height; PE = mgh. Calculate for different gravity of different enviornments - Earth, the Moon, Jupiter, or specify your own. Free online physics calculators, mechanics, energy, calculators.
Explanation:
Which of the following has the highest boiling point? Hint: They are all non polar. CH4 CH3CH3 CH3CH2CH3 CH3CH2CH2CH3
CH3CH2CH2CH3
Answers
CH3CH2CH2CH3 i.e., butane having more boiling point among the all given .
Compounds composed of molecules joined by chemical bonds arranged so that their charge distributions are symmetrical are called non-polar compound
Compounds composed of molecules joined by asymmetric polar bonds.
Polar compounds exhibit polarity. This is a property characterized by two opposite charges or poles. As such, it can be converted into a water-soluble ion, which is a polar molecule.
Reason :
while increasing the number of carbons in the chain, the boiling point also increases. The alkanes having intractions with the neighbour alkanes will be Van der Waals dispersion forces, these intractions are very less in the small molecule such as methane. The the force of attraction between the molecules increases as the molecule gets longer and has more electrons.
Learn more about Polar and Non-polar compound here :
https://brainly.com/question/1426521
#SPJ4
Give me some examples of why is it important to understand how ocean currents flow.
Answers
Answer:
By moving heat from the equator toward the poles, ocean currents play an important role in controlling the climate. Ocean currents are also critically important to sea life. They carry nutrients and food to organisms that live permanently attached in one place, and carry reproductive cells and ocean life to new places.
Explanation:
:)
What medal has the highest volume?
Answers
The Medal of Honor is the best navy decoration and highest volume.
The President of the USA The Victoria go is the holy grail for Medal of Honor creditors because there are the best in lifestyles. Bearing the inscription For valor and called a VC, this medal turned into first offered for conspicuous bravery' in 1856 and later backdated to the Crimean conflict of 1854. The outstanding service pass is the second maximum army ornament that may be provided to a member of the American military, for intense gallantry and risk of lifestyle in actual combat with an armed enemy force.
The Bronze Star Medal dates lower back to world battle II. these days, it is the fourth-maximum ranking award a provider member can obtain for a heroic and meritorious deed performed in an armed battle. For individuals who acquire the BSM, it's far a sign of their sacrifice, bravery, and honor at the same time as serving their us of a
Learn more about medals here:-https://brainly.com/question/17634999
#SPJ9
milk has a pH of 6.0 and household ammonia has a pH of 12.0. How much more acidic is milk than ammonia
Answers
60 I think bcz if there is 1&2 they differ 10 times
The milk has a pH of 6.0 and household ammonia has a pH of 12.0. then milk will be 6 times milk acidic than ammonia.
What is pH?The pH scale, which previously stood for "potential of hydrogen," would be used to describe how acidic or basic an aqueous solution is.
What is an acidic solution?More hydrogen ions are present in an acidic solution than it was in pure water.
It is given then,
\((milk)pH_{1} = 6\\(ammonia)pH_{2} = 12\)
The concentration of hydrogen ions can be calculated by using the formula:
\([H^{+}] = 10^{-pH}\)
By putting the value of given data in the above equation.
\([H^{+}_{milk} ] / H^{+}_{ammonia}= 10^{-6}/10^{-12}\\ = 10^{6} = 6\)
We get that, milk will be 6 times more acidic than ammonia.
To know more about pH and acidic solutions
https://brainly.com/question/15289741
#SPJ2
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution that freezes at −10.0°C? Assume the density of water is 1.0 g/mL. Kf of water is 1.86°C/m.
Answers
Answer: Thus 2473 g of ethanol must be added to 10.0 L of water to give a solution that freezes at −10.0°C
Explanation:
Depression in freezing point is given by:
\(\Delta T_f=i\times K_f\times m\)
\(\Delta T_f=T_f^0-T_f=(0-(-10))^0C=10^0C\) = Depression in freezing point
i= vant hoff factor ( for non electrolytes , i= 1)
\(K_f\)= freezing point constant for water= \(1.86^0C/m\)
m= molality
\(\Delta T_f=i\times K_f\times \frac{\text{mass of solute}\times 1000}{\text{molar mass of solute}\times \text{weight of solvent in g}}\)
weight of solvent (water ) = \(density\times Volume\)
weight of solvent (water) =\(1.0g/ml\times 10000ml=10000g\) ( 1L=1000ml)
\(10^0C=1\times 1.86^0C/m\times \frac{x\times 1000}{46\times 10000g}\)
\(x=2473g\)
Thus 2473 g of ethanol must be added to 10.0 L of water to give a solution that freezes at −10.0°C
HELP ME PLSSSSSSSSSSSSAA

Answers
Answer:
If capital R is round, than the RR and Rr will mean round, and the two rr's are wrinkled. that means the answer to both is 50%
Explanation:
hope that helps
Consider a gradient elution run of non-polar compounds in reverse
phase HPLC with a methanol-water gradient. Does the mobile phase
start as pure water and then end up as pure methanol or is it done the
other way? Explain your reasoning fully.
Answers
The order of elution in reversed-phase HPLC is different from that in a normal-phase separation, with more polar solutes eluting first. The mobile phase's polarity is increased to produce longer retention periods.
What is HPLC ?A method in analytical chemistry called high-performance liquid chromatography, formerly known as high-pressure liquid chromatography, is used to separate, recognize, and quantify each component in a mixture.
To obtain a consistent increase in the organic solvent (usually methanol or acetonitrile) over the course of the study, gradients in reversed-phase HPLC typically use on-line (dynamic) mixing of solvents. This increases the elution strength of the eluent over time.
Thus, In contrast to the normal phase HPLC, which employs a polar stationary phase and a less polar mobile phase, the reverse phase HPLC makes use of a nonpolar stationary phase and a polar mobile phase.
To learn more about HPLC, follow the link;
https://brainly.com/question/2292345
#SPJ1
Choose all the right answers.
When the leaf of a fern touches the ground, it may produce a new plant:
by planting a spore
by vegetative reproduction
by growing roots at the point of contact
by budding from its stem
Answers
Answer:
I think by planting a spore
Explanation:
jsjsbfhdkdnsofi
Which of the following correctly describes a mixture?
Answers
A mixture can be defined as a physical blend of two or more substances that are not chemically combined.
They retain their own properties and can be separated by physical means like filtration, distillation, evaporation, or magnetism. The various types of mixtures include homogeneous mixtures, heterogeneous mixtures, and colloids.Homogeneous mixtures, also known as solutions, are uniform mixtures where the composition is the same throughout. They are not visibly different and consist of a solute (the substance being dissolved) and a solvent (the substance doing the dissolving). For example, salt water is a homogeneous mixture because the salt is dissolved uniformly throughout the water.Heterogeneous mixtures are non-uniform mixtures that consist of two or more phases, each with its own distinct properties. They can be seen with the eye, and the different components can be separated using physical means. An example of a heterogeneous mixture is oil and water. They can be mixed together, but they will eventually separate.Colloids are mixtures where the particle size is intermediate between that of a solution and a suspension. The particles are small enough to not be visible to the eye, but they are large enough to scatter light. Milk is an example of a colloid because it appears homogeneous but is actually made up of small particles of fat and protein dispersed throughout the liquid.In conclusion, a mixture is a physical blend of two or more substances that are not chemically combined. They can be separated by physical means and consist of homogeneous mixtures, heterogeneous mixtures, and colloids.
for such more questions on substances
https://brainly.com/question/29108029
#SPJ8