Q21 90g of glucose is dissolved in water. The glucose solution is fermented. C6H12O62CO₂ + 2C₂H₂OH glucose, M, = 180 After the fermentation finishes, 6.8g of ethanol is obtained from the solution. What is the percentage yield of ethanol? A 7.4 B 7.6 C 14.8 ethanol, M, = 46 D 29.6
Answers
The correct answer is 14.8%.
The given reaction,
C_6H_12O_6 ----> 2CO₂ + 2C₂H₂OH
A key idea in chemistry is stoichiometry, which determines the quantity of reactants or products in a chemical reaction and aids in understanding different reactions and the proportions in which they might take place. A combination of two Greek terms is stoichiometry. Stoicheion, which denotes an element, and metron, which denotes a unit of measurement
mole= mass/mole mass
=90g/180g/mol
=0.5mol
According to stoichiometry
1 mol glucose = 2 mol ethanol
0.5 mol C_6H_12O_6 = 1 mole ethanol
mass of ethanol = 1 mole ×46 g/ mol
Theoretical yield = 46 g
percentage = actual yield / theoretical yield × 100
=6.8 g / 46 g × 100 = 14.78% =14.8%
To learn more about percentage yield refer the link:
https://brainly.com/question/15443303
#SPJ9
Related Questions
An aluminum can is cut into small pieces. A 1. 16-g sample of the aluminum chips is used to prepare potassium alum according to the procedure described in this experiment. Calculate the theoretical yield (in grams) of potassium alum that could be obtained in the reaction using the correct number of significant figures. The molar mass of potassium alum is 474. 39g/mol.
Answers
To calculate the theoretical yield of potassium alum, we need to determine the number of moles of aluminum present in the 1.16 g sample and then use the stoichiometry of the reaction to find the corresponding number of moles of potassium alum.
Therefore, the theoretical yield of potassium alum that could be obtained in the reaction is approximately 10.23 grams.
First, we calculate the number of moles of aluminum using its molar mass:
Number of moles of aluminum = Mass of aluminum / Molar mass of aluminum
= 1.16 g / 26.98 g/mol (molar mass of aluminum)
≈ 0.043 moles
Next, we use the balanced chemical equation for the reaction between aluminum and potassium alum to find the mole ratio between aluminum and potassium alum. The balanced equation is:
2 Al + K2SO4 · Al2(SO4)3 + K2SO4
From the balanced equation, we see that 2 moles of aluminum react to form 1 mole of potassium alum.
Therefore, the theoretical yield of potassium alum is:
Theoretical yield = Number of moles of aluminum * (1 mole of potassium alum / 2 moles of aluminum)
= 0.043 moles * (1 mole / 2 moles)
= 0.0215 moles
Finally, we convert the number of moles of potassium alum to grams using its molar mass:
Theoretical yield in grams = Theoretical yield in moles * Molar mass of potassium alum
= 0.0215 moles * 474.39 g/mol (molar mass of potassium alum)
≈ 10.23 g
Therefore, the theoretical yield of potassium alum that could be obtained in the reaction is approximately 10.23 grams.
Learn more about reaction here
https://brainly.com/question/16737295
#SPJ11
What is the formula for this ionic crystal?

Answers
Answer: I'm willing to help you find the formula for that ionic crystal but, I can't see anything.
The ionic crystal contains a total of 6 Ba atoms and 6 oxygen atoms. Hence they are in 1 : 1 ratio and the formula of the ionic compound is BaO.
What are ionic compounds?Ionic compounds are formed between metals and non-metals. Ionic bond is formed by the lose of electrons from the metals to the nonmetals. The chemical formula of the ionic compounds are written with the cationic symbol first.
The given ionic crystal is formed with barium and oxygen atoms. Valency of barium is 2 and that of oxygen is also 2. Thus, barium loses two electrons to oxygen forming BaO.
The given crystal lattice consists of 3 barium atoms and 3 oxygen atoms in front face and 3 Ba and 3 o in back face also. Thus, both are 6. They are in the ratio of 1 : 1. Hence, the compound is named as BaO.
To find more on ionic compounds, refer here:
https://brainly.com/question/17217225
#SPJ5
Which element is in the noble gas family? A. Iodine (I)
B. Nitrogen (N)
C. Radon (Rn)
D. Oxygen (O)
Answers
Answer:
Radon
Explanation:
:
Answer:
c. Radon (Rn)
Explanation:
hope this can help
The free energy change for a reaction, ΔG, depends on the logarithm of the equilibrium constant Keq. A large negative value of ΔG therefore translates to a _____ value of Keq and a _____ difference in the relative amounts of starting materials vs products at equilibrium.
Answers
A large negative value of ΔG, therefore, translates to a large value of Keq and a small difference in the relative amounts of starting materials vs products at equilibrium.
A large negative value of ΔG corresponds to a large value of Keq, indicating that the products are favored at equilibrium over the starting materials. In other words, a large negative ΔG implies that the reaction proceeds almost entirely in the forward direction, and there is very little starting material left at equilibrium.
Conversely, a small or positive value of ΔG corresponds to a small or negligible value of Keq, indicating that the starting materials are favored over the products at equilibrium. In other words, the magnitude and sign of ΔG determine the direction and extent of the reaction, and whether it is energetically favorable or unfavorable.
Learn more about negative ΔG here:
https://brainly.com/question/639438
#SPJ11
A 1.2 M solution of H2S reacts with a 0.80 M solution of NH2-. What volume of H2S is needed to neutralize 45mL of NH2-?
Answers
The volume of H2S needed to neutralize 45mL of NH2- is 0.053 L
What do you mean by neutralization reaction?A neutralization reaction, in which an acid and a base react to form water and salt.
The reaction between H2S and NH2- is a neutralization reaction. The balanced equation for the reaction is:
H2S(aq) + NH2-(aq) → NH4HS(aq)
We know that the concentration of H2S is 1.2 M and the concentration of NH2- is 0.80 M and we are trying to find the volume of H2S needed to neutralize 45mL of NH2-.
The number of moles of H2S can be calculated by using the formula:
moles = molarity x volume
The volume of H2S needed to neutralize 45mL of NH2- is equal to the number of moles of NH2- in 45mL, divided by the concentration of H2S.
moles of NH2- = 0.080 L * 0.80 mol/L = 0.064 mol
volume = moles of NH2- / molarity of H2S
volume = 0.064 mol / 1.2 mol/L = 0.053 L
Therefore, the volume of H2S needed to neutralize 45mL of NH2- is 0.053 L.
To know more about neutralization, visit:
brainly.com/question/27891712
#SPJ1
wafting the air above a chemical is one way to it directly
Answers
Answer:
Explanation:
All it takes is a momentary lapse in concentration. When you are in the laboratory and take a direct sniff of the chemicals you are using, you run the risk of damaging your mucous membranes or your lungs.
Quadrilaterals Test
really need help
grades are low
pls and ty :)
Answers
Quadrilaterals are polygons with four sides, and studying their properties and classifications will help you identify and analyze various types of quadrilaterals effectively.
Understanding quadrilaterals is essential for geometry, and improving your grades in this area is achievable with guidance. A quadrilateral is a polygon with four sides. By studying the properties of quadrilaterals, you can identify and analyze different types of quadrilaterals. Key properties include angles, side lengths, and polygons . Some common types of quadrilaterals include squares, rectangles, parallelograms, trapezoids, and rhombuses. Each type has specific properties and characteristics that distinguish it from others. Learning these properties will help you classify and solve problems related to quadrilaterals. By practicing identifying and working with polygons , you can improve your understanding and performance in this topic. If you have specific questions or need further assistance, feel free to ask.
To learn more about polygons, click here:
brainly.com/question/23846997
#SPJ11
Determine the mass of aspirin produced when 150.0 g of salicylic acid reacts with an excess of acetic anhydride.
Answers
The mass of aspirin produced when 150.0 g of salicylic acid reacts with an excess of acetic anhydride is 196.0 g.
When salicylic acid reacts with acetic anhydride, it forms aspirin and acetic acid. The balanced chemical equation for this reaction is:
C7H6O3 + (C2H3O)2O → C9H8O4 + CH3COOH
From this equation, we can see that one mole of salicylic acid reacts with one mole of acetic anhydride to produce one mole of aspirin and one mole of acetic acid. The molar mass of salicylic acid is 138.12 g/mol and the molar mass of aspirin is 180.16 g/mol.
To determine the mass of aspirin produced when 150.0 g of salicylic acid reacts with an excess of acetic anhydride, we first need to calculate the number of moles of salicylic acid present in the reaction:
n = m/M = 150.0 g / 138.12 g/mol = 1.086 mol
Since there is an excess of acetic anhydride present in the reaction, we know that all of the salicylic acid will react to form aspirin. Therefore, the number of moles of aspirin produced will also be 1.086 mol.
Finally, we can calculate the mass of aspirin produced by multiplying the number of moles by the molar mass:
m = n x M = 1.086 mol x 180.16 g/mol = 196.0 g
Therefore, the mass of aspirin produced when 150.0 g of salicylic acid reacts with an excess of acetic anhydride is 196.0 g.
To know more about aspirin visit:
https://brainly.com/question/14988384
#SPJ11
In the set up below, which air should show an increase in temperature?

Answers
Answer:
My guess is A.
Explanation:
Dorothy left a frozen juice pop out on the counter. When she returned, the juice pop had melted into a liquid. Which of the following is true of the molecules in the juice pop?
Group of answer choices
The molecules had more kinetic energy when the juice pop was frozen.
The molecules were closer together when the juice pop was frozen.
The molecules became more tightly packed when the juice pop melted.
The molecules moved less freely when the juice pop melted.
Answers
Answer:
B) The molecules were closer together when the juice pop was frozen.
A student described two properties of a substance as shown. Properties of Substance Property Description C A known mass of the substance gives off heat when it is burned. D A substance can be stretched out to become a long wire. Which of the following is true about the two properties described in the table? (5 points) Both are physical properties. Both are chemical properties. C is a chemical property and D is a physical property. C is a physical property and D is a chemical property.
Answers
The change in size is a physical property and and the heat evolution by burning is a chemical property. Hence, option c is correct.
What are physical properties ?Physical properties of a substance involves, change in state, size or shape. They does not involve formation of a new compound and thus does nit undergo breaking or making of chemical bonds.
A chemical change involves the breaking or making of chemical bonds results in the formation of a new product. The change in size of a material by stretching it is a physical change.
Burning a material involves breaking of bonds and the formation of ash and other side products. Hence, option c is correct. C is a chemical property and D is a physical property.
Find more on physical properties:
https://brainly.com/question/18327661
#SPJ1
Which radioisotope is naturally occurring? Question 3 options: a. 24296Cm b. 31H c. 258103Lr d. 23894Pu
Answers
(d) 23894Pu is the radioisotope that is found naturally. It is a naturally occurring element that is present in uranium ores in very small concentrations.
Except 23894Pu, which is found naturally, the remaining isotopes on the list, in contrast, are typically created in nuclear reactors or particle accelerators and are not naturally occurring.
A. synthetic isotope created by nuclear processes is 24296Cm.
B. The radioactive hydrogen isotope 31H is a synthetic isotope.
C. The isotope 258103Lr is artificial, has a very brief half-life, and is not naturally occurring on Earth.
The term "radioisotope" refers to an unstable isotope of an element that undergoes radioactive decay. Isotopes are atoms of the same element with variable numbers of neutrons but the same number of protons in their nucleus. Nuclear reactions can be used to create radioisotopes intentionally or naturally in the environment. They are used in a wide variety of scientific, medical, industrial, and agricultural fields.
To know more about radioisotopes, refer:
https://brainly.com/question/13213627
#SPJ4
PbS+FeN → Fe2S3 + Pb3N2
Can someone please valence this for meeeee
Answers
Answer:
html
Explanation:
im in web tech
in lower latitudes where a thermocline exists, as we drop down from the surface through ocean water, water density will increase while water temperature will respond inversely.
Answers
In the lower latitudes where thermocline will exists, as we drop down from surface through ocean water, water density will increase while water temperature will generally decrease.
A thermocline refers to a layer in the ocean where there is a rapid change in temperature with depth. In lower latitudes, especially in tropical regions, there is a pronounced thermocline due to the heating of surface waters by sunlight and the subsequent stratification of water layers.
As we descend through the ocean water from the surface, the water density tends to increase. This is because colder water will be denser than warmer water. In the surface layer, which is exposed to sunlight and warmer air temperatures, the water is generally warmer and less dense. However, as we move deeper into the thermocline, the water becomes colder and denser due to factors like mixing, upwelling, or the influence of cooler currents.
Conversely, the water temperature will generally decrease as we go deeper into the thermocline. The warmest water is found near the surface, where it is exposed to solar radiation and warmer air temperatures.
To know more about thermocline here
https://brainly.com/question/953795
#SPJ4
--The given question is incomplete, the complete question is
"In lower latitudes where a thermocline exists, as we drop down from the surface through ocean water, water density will ___________while water temperature will respond inversely."--
calculate the enthalpy for the reaction D + F = G + H using
G + C = A + B deltaH = 277
C + F = A delta H = 303
D = B + H delta H = -158
Answers
The enthalpy for the reaction : ΔH = -132
Further explanationGiven
Reaction and the enthalpy
Required
the enthalpy
Solution
Hess Law
Reaction 1 reverse :
A + B = G + C ΔH = -277
Reactions 2 and 3 remain the same (unchanged)
C + F = A ΔH = 303
D = B + H ΔH = -158
Add up all the reactions and remove the same compound from two different sides
D + F = G + H ΔH = -132
CHEMISTRY EXCERCISES

Answers
1. (a) Class: carboxylic acid; IUPAC name: propanoic acid. (b) Class: alkyl halide; IUPAC name: chloro-1-propane. (c) Class: alkane; IUPAC name: 1-propanecarbonitrile. (d) Class: ester; IUPAC name: ethyl methanoate.
What is IUPAC name?IUPAC stands for International Union of Pure and Applied Chemistry. It is an international scientific organization responsible for developing and promoting international standards in the fields of chemistry and chemical nomenclature. The IUPAC name of a chemical compound is an unambiguous, systematic method for naming compounds according to their chemical structure and physical properties.
(e) Class: ether; IUPAC name: dimethyl ether. (f) Class: acyl halide; IUPAC name: 1-chloro-2,2-difluoropropane-1-carbonyl chloride.
2. (a) Hexanoic acid: CH3CH2CH2CH2CH2COOH. (b) Butanal: CH3CH2CHO. (c) Pent-1-ene: CH2=CHCH2CH2CH3. (d) 1-bromo-2-methylbutane: CH3CH2CH(Br)CH3. (e) Ethyl methanoate: CH3COOCH2CH3. (f) Methoxypropane: CH3OCH2CH3. (g) But-2-yne: CH3C≡CHCH3.
3. Answer: B. CH3CONH2 is an amine because it contains an amine group (NH2).
4. Answer: A. 1-iodopropane is a member of the same homologous series as 1-bromopropane because they both have the same molecular formula (C3H7Br or C3H7I) and the same functional group (halogen).
To learn more about IUPAC name
https://brainly.com/question/28872356
#SPJ1
286 L of hydrogen gas at STP reacts with excess chorine gas to make hydrogen chloride gas (HCl). What is the maximum amount of gas product that can be formed at STP
Answers
The maximum amount of gas product (HCl) that can be formed at STP is 573.44 L.
Hydrogen gas (H₂) at STP occupies a volume of 22.4 L per mole. So, 286 L of hydrogen gas at STP is 286/22.4 = 12.8 moles of hydrogen gas. The balanced chemical equation for the reaction of hydrogen gas and chlorine gas (Cl₂) is:
H₂ + Cl₂ → 2HCl
1 mole of H₂ reacts with 1 mole of Cl₂ to form 2 moles of HCl.
12.8 moles of H₂ would require 12.8 moles of Cl₂ for complete reaction.
However, the question states that there is excess chlorine gas, so the hydrogen gas would be the limiting reactant.
Hence, the maximum amount of gas product that can be formed is 2 x 12.8 = 25.6 moles of HCl.
At STP, 1 mole of any ideal gas occupies a volume of 22.4 L.
So, 25.6 moles of HCl would occupy a volume of 25.6 x 22.4 = 573.44 L.
Therefore, the maximum amount of gas product (HCl) that can be formed at STP is 573.44 L.
To know more about gas product visit:
https://brainly.com/question/14626988
#SPJ11
How many grams of lithium are in 3.50 moles of lithium
Answers
convert 2.5 X 10^24 atoms of copper to grams of copper
Answers
2.5 X 10²⁴ atoms of copper is equivalent to 266.7g of Cu.
HOW TO CALCULATE MASS:
The mass of a substance can be calculated by multiplying the number of moles of the substance by its molar mass. That is;mass of substance (g) = no. of moles (mol) × molar mass (g/mol)However, the number of moles of copper must first be calculated by dividing the number of atoms by Avogadro's number as follows:no. of moles of Cu = 2.5 X 10²⁴ ÷ 6.02 × 10²³no. of moles = 2.5/6.02 × 10²⁴-²³no. of moles = 0.42 × 10¹no. of moles = 4.2moles of copper. Molar mass of copper = 63.5g/molMass of copper = 4.2mol × 63.5g/molMass of copper = 266.7gTherefore, 2.5 X 10²⁴ atoms of copper is equivalent to 266.7g of Cu.
Learn more at: https://brainly.com/question/12154684?referrer=searchResults
Please help with this. Very much appreciated thank you so much.
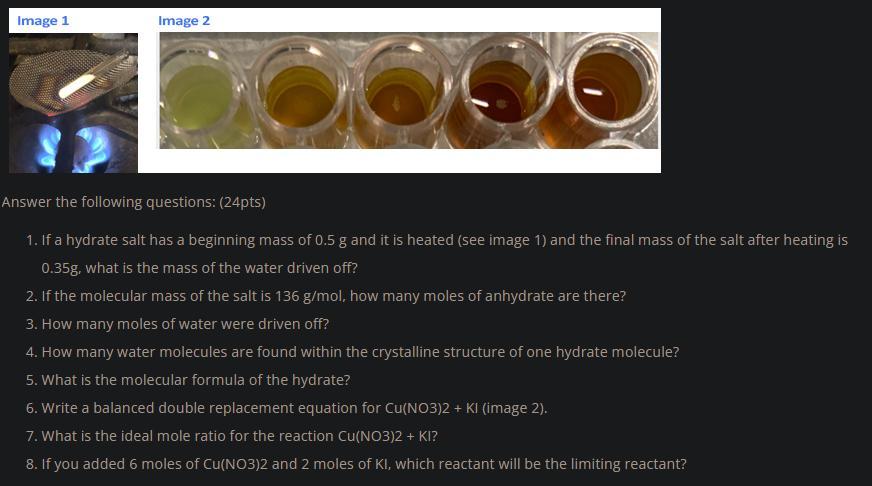
Answers
Mass of water driven off = 0.15 g
Moles of anhydrate 0.00257 moles
Moles of water driven off = 0.00833 moles
Moles of water per mole of hydrate = 3 moles
Molecular formula of the Hydrate is X.3H₂O where X is the anhydrate salt.
Cu(NO₃)₂ + 2 KI ---> 2 KNO₃ + CuI₂
The ideal mole ratio is 1 : 2
The limiting reactant will be KI.
What is the mass of water driven off?A hydrate salt is salt that contains water of crystallization.
The mass of water driven off = 0.5 - 0.35 g
Mass of water driven off = 0.15 g
Moles of anhydrate = 0.35/136
Moles of anhydrate = 0.00257 moles
Moles of water driven off = 0.15/18
Moles of water driven off = 0.00833 moles
Moles of water per mole of hydrate = 0.00833/0.00257
Moles of water per mole of hydrate = 3 moles
Molecular formula of the Hydrate is X.3H₂O where X is the anhydrate salt.
The double replacement equation is given below:
Cu(NO₃)₂ + 2 KI ---> 2 KNO₃ + CuI₂
The ideal mole ratio for the reaction of Cu(NO₃)₂ and KI is 1 : 2
If 6 moles of Cu(NO₃)₂ was added to 2 moles of KI, the limiting reactant will be KI.
In conclusion, a hydrated salt is a salt that contains water of crystallization.
Learn more about hydrates at: https://brainly.com/question/19535956
#SPJ1
A student combines 364.6 g of HCl with 80 g of NaOH in 5 L of water. What additional volume of H2O must be added to this mixture to yield a solution with a pH of 1? Note that the molar mass of HCl is 35.46 g/mole, while that of NaOH is 40 g/mole.
Answers
Answer:
75L of additional water to have a pH 1 solution
Explanation:
The reaction of HCl With NaOH is:
HCl + NaOH → H₂O + NaCl
By using molar mass of each reactant you can know how many moles will react, thus:
HCl: 364.6g HCl ₓ (1mol / 36.46g) = 10 moles HCl
NaOH: 80g NaOH ₓ (1mol / 40g) = 2 moles NaOH
That means after the reaction will remain in solution, 10-2 = 8 moles of HCl = 8 moles of H⁺ (In water, HCl dissociates as H⁺ and Cl⁻ ions).
A solution with pH = 1 contains:
pH = -log [H⁺]
1 = -log [H⁺]
0.1M = [H⁺]
As molarity, M is the ratio between moles and liters and you want a solution 0.1M having 8 moles of H⁺ you require:
0.1M = 8 moles H⁺ / 80L
As the student combines the solution with 5L of water, you require
75L of additional water to have a pH 1 solutionplease help what's the meaning of ato.s and list the parts
Answers
Explanation
Structure Of The Atom Our current model of the atom can be broken down into three constituents parts protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive charge electrons having a negative charge and neutrons possessing no net charge.
How many chlorine molecules are in 6.5 moles of chlorine?

Answers
Answer:
the number of chlorine molecules in 6.5 moles of chlorine is calculated as follows
by use of Avogadro constant that is 1mole = 6.02 x10^23 molecules
what about 6.5 moles
6.5 x ( 6.02x 10^23)= 3.913 x10^24
ANSWER ASAP GIVING BRAINLIEST FIVE STARS AND A HEART!
How are thermal energy and heat related?
Answer in your own words using 5-7 sentences!
Answers
Answer:
Nothing
Explanation:
Thermal energy and heat are mainly related because of the fact that the faster molecules move, the more heat is created. If molecules are vibrating slower, that means that there will be less energy, resulting in less heat. Say I am boiling water. As the temperature increases, the water molecules move faster. Once that water cools, the molecules will be moving really slowly. In conclusion, thermal energy and heat are related because of how fast or slow the molecules move, the more or less heat and energy will be produced. I hoped this helped!
How many significant figures are in 20600?
Answers
There are 3 significant figures in 20600.
1. For each statement, circle T
or F for true or false. In each
blank, write the number of the
SENTENCE that gives the best
evidence for the answer.
a. Frogs, lily pads, and fish are
parts of a pond community.
T. Or. F. Sentence ________
b. An ecosystem includes only a
living things.
T. Or. F. Sentence ________
c. All the barn owls in a state
park make up a population of
owls.
T. Or. F. Sentence ________
d. Squirrels and mice are two
different populations of
animals.
Τ. Or. F. Sentence ________
e. A desert community is made up
of cactus, sand, and camels.
Τ. Or. F. Sentence ________
f. A desert ecosystem can
include cactus, sand, and
camels.
T. Or. F. Sentence ________
2. What is the most likely
meaning of interact as it is
used in sentence 8?
a. affect each other
b. grow together
c. reproduce
d. breathe
Write the number of the other
sentence (not sentence 8) that
gives the best evidence for the
answer.
3. Name an ecosystem.
__________________________
List 5 populations of organisms you
would expect to find in it
___________________________
___________________________
___________________________
___________________________
___________________________
4. A taiga is an ecosystem that
is cold in the winter, cool in
the summer, has little rainfall,
and supports evergreen trees,
moose, and weasels. Which other
ecosystem does it most resemble?
__________________________
5. Complete the diagram below
to compare the populations in
the desert and the rain forest
ecosystems.
COMPARING ECOSYSTEMS
Reason for comparing:
Know more about the populations
What is being compared?
____________ vs ___________
How same?
___________
___________
___________
___________
VS.
How different?
___________
___________
___________
___________
Conclusion
____________________no

Answers
A taiga is an ecosystem that is cold in the winter, cool in the summer, has little rainfall, and supports evergreen trees, moose, and weasels, this ecosystem most resembles the boreal forest ecosystem.
The ecosystema. Frogs, lily pads, and fish are parts of a pond community.
True. Sentence 3.
b. An ecosystem includes only living things.
False. Sentence 4.
c. All the barn owls in a state park make up a population of owls.
True. Sentence 2.
d. Squirrels and mice are two different populations of animals.
True. Sentence 6.
e. A desert community is made up of cactus, sand, and camels.
False. Sentence 5.
f. A desert ecosystem can include cactus, sand, and camels.
True. Sentence 8.
2. The most likely meaning of interact in sentence 8 is option a. affect each other.
Sentence 9 gives the best evidence for this answer.
3. An example of an ecosystem is the Amazon Rainforest.
5 populations of organisms you would expect to find in it are:
JaguarsToucansSlothsMacawsTapirs4. A taiga is an ecosystem that is cold in the winter, cool in the summer, has little rainfall, and supports evergreen trees, moose, and weasels.
The taiga ecosystem most resembles the boreal forest ecosystem.
COMPARING ECOSYSTEMS
What is being compared is:
Desert vs Rainforest
Their similarities are:
Both have diverse plant and animal species.Both are ecosystems.Their differences are:
Desert has sparse vegetation, while rainforest has dense vegetation.Desert has extreme temperature variations, while rainforest has a relatively stable climate.Desert has limited water availability, while rainforest has high rainfall.In conclusion, the desert and rainforest ecosystems differ significantly in terms of vegetation, climate, and water availability.
Learn more on the ecosystem here https://brainly.com/question/842527
#spj1
1.
a. True. Sentence 1.
b. False. Sentence 2.
c. True. Sentence 3.
d. True. Sentence 4.
e. False. Sentence 5.
f. True. Sentence 6.
2. The most likely meaning of interact as it is used in sentence 8 is "affect each other." Sentence 7 provides the best evidence for this answer, stating that "the living and nonliving things in an ecosystem depend on each other."
3. Rainforest is an ecosystem. Five populations of organisms that can be found in a rainforest include:
TreesBirdsInsectsReptilesAmphibians4. A taiga ecosystem most resembles a tundra ecosystem.
5. The following diagram compares the populations in the desert and the rain forest ecosystems:
COMPARING ECOSYSTEMS
Reason for comparing: Know more about the populations
What is being compared?We are comparing Desert vs. Rain forest
How same?
Firstly, the dryness typical of desert conditions characterize these environments alongside sparse rainfall which contrast significantly with the constant wetness experienced in the tropical setting of the Rainforest regime . This also reflects on temperatures as while desert areas experience scorching heat during daytime periods, they also have incredibly chilly nights unlike areas inhabited by Rainforests which tend to maintain moderate- warm daytime climate tempered by cool nighttime weather. They also differ strikingly when it comes to soil texture ;desert soils lean toward sandiness whereas those found within tropical regions such as Rainforests tend to possess nutrient-rich quality.Habitat wise, Rainforests typically host a diverse range of tree species while deserts have fewer to no trees.Learn about ecosystem here https://brainly.com/question/30187156
#SPJ1
Scenario 1: Sarah, your lab partner, accidentally poured too much
sodium chloride (chemical) into a beaker. She wants to pour the rest back
into the original container. What should you do? Explain
Scenario 2: You notice that yout lab partner is chewing gum and using a
beaker as a cup to get water. He then takes a drink from the beaker. He
assures you that it is clean and you don't have to worry. Why is this
dangerous in lab? Explain.
Scenario 3: In lab, you are working with an open flame (a birthday
candle) and your friend calls you over to see new background picture on
his chromebook. What should you do? Explain
Answers
Scenario 2: chemicals have been on beakers and they should not be drank out of, your partner should also not be eating or drinking in the lab.
Scenario 3: you should never walk away from an open flame
why do the noble gases have relatively low electron affinities?
Answers
Answer:
Because noble gases have a full octet, their ionization energies are actually quite high. Electron affinity is the amount of energy released when an electron is added to a neutral atom. Because noble gases have stable electron configurations, they have very low electron affinities.
Explanation:
Answer:
Noble gases have a stable outer shell
Explanation:
Sucrose is very soluble in water. At 25◦C,
211.4 grams of sucrose will dissolve in 100
g of water. Given that the density of the
saturated sucrose solution is 1.34 g/mL, what
is the molarity of the solution? The molar
mass of sucrose is 342 g/mol.
(It's not 1 or 3)

Answers
Answer:
number of moles of solute dissolved in one liter of solution.1. 8.28
Explanation:
I'm not sure but I hope it's help
At the energy-generating station, the coal is burned. Burning
changes, the coal's chemical energy into sound energy.
true or
false
Answers
Answer:
The correct answer is - false.
Explanation:
At the energy-generating station, when the coal is burned, the chemical energy store in the coal converted into thermal energy and light or luminous energy.
The thermic energy generated used to heat and boil the water or liquid to generate the gas and generate the kinetic energy to move the fan that is desgined to generate the electrical energy.
Thus, the statment is false statement as it does not convert into sound energy.