Pureguardian 120-hour 2-gallon ultrasonic warm and cool mist humidifier with aroma tray.
Answers
The Pureguardian 120-hour 2-gallon ultrasonic humidifier is a device that releases both warm and cool mist. It also comes with an aroma tray.
The Pureguardian 120-hour 2-gallon ultrasonic warm and cool mist humidifier with aroma tray is a humidifier that releases both cool and warm mist into the air. This device has a 2-gallon tank capacity that can provide up to 120 hours of continuous use. The ultrasonic technology it uses ensures that the mist is dispersed evenly throughout the room.The warm mist setting is useful during the winter months as it can provide warmth to the room.
The device also comes with an aroma tray, which can be used to add essential oils to the mist for added aromatherapy benefits. The Pureguardian humidifier also features Silver Clean Protection technology that helps to prevent the growth of mold and mildew in the tank.
Learn more about aromatherapy here:
https://brainly.com/question/30899693
#SPJ11
Related Questions
Determine to which elements the given atomic masses belong:
1.708 × 10 -25 kg
Answers
Answer: The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon-12 atom considering that it is at rest.
If a 1.0x10-5 L sample of agas at 2.0x106 atm isreleased until it is equal to0.275 atm, what would thenew volume of the gas be?
Answers
Answer:
Explanation:
Here, we want to get the new volume of the gas
What we need to know is the law that connects volume and pressure at constant temperature
The law that supports this is the Boyle's law
It states that the volume of a given mass of gas is inversely proportional to its pressure at constant temperature
Mathematically, we can have this as represented as:
\(\begin{gathered} P_1V_1=P_2V_2 \\ P_1\text{ = 2.0 }\times10^{6\text{ }}\text{ atm} \\ V_1\text{ = 1.0 }\times10^{-5}\text{ L} \\ P_2\text{ =0.275 atm} \\ V_2\text{ = ?} \end{gathered}\)We can proceed mathematically to solve as follows;
\(undefined\)I need help getting this done
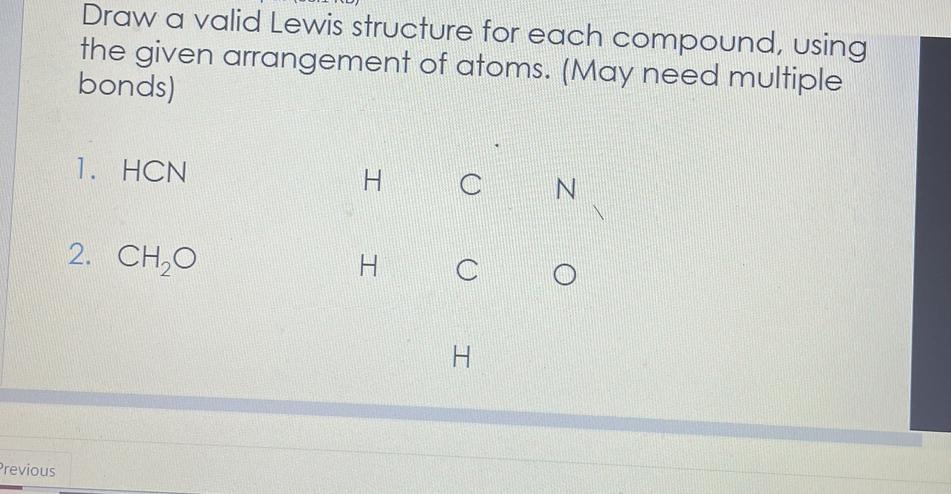
Answers
Answer:
I put the right structures in the pictures for you


What volume in liters will 50.0g CO occupy? please show work.
Answers
Answer:
40.0L is the volume the CO occupy
Explanation:
To solve this question we must assume the gas is at STP. To find the volume of a gas using the moles, temperature and pressure of the gas we have to use:
PV = nRT
V = nRT / P
Where V is volume in liters
n are moles of the gas -Molar mass CO: 28g/mol-
50.0g * (1mol / 28g) = 1.786 moles CO
R is gas constant = 0.082atmL/molK
T is absolute temperature = 273.15K at STP
P is pressure = 1atm at STP
Replacing:
V = 1.786moles*0.082atmL/molK*273.15K / 1atm
V = 40.0L is the volume the CO occupy
Nitric oxide forms during combustion. N2 (g) + 02 (g) = 2NO (g) The
concentrations at equilibrium at a certain temperature are
[N2] = 0.5 mol / L [02] = 0.3 mol / L [NO] = 0.10 mol / L
Calculate the Keg for the reaction.
Answers
The equilibrium constant, Keq for the reaction given that [N₂] = 0.5 mol/L, [O₂] = 0.3 mol/L, and [NO] = 0.10 mol/L is 0.067
How do I determine the equilibrium constant, Keq?Equilibrium constant, Keq for a given reaction is by the following formula:
nReactant ⇌ mProduct
Equilibrium constant (Keq) = [Product]ᵐ / [Reactant]ⁿ
Now, we can shall determine the equilibrium constant, Keq for the reaction. This is illustrated below:
N₂(g) + O₂(g) ⇌ 2NO(g)
Concentration of nitrogen [N₂] = 0.5 mol/LConcentration of oxygen [O₂] = 0.3 mol/LConcentration of nitric oxide [NO] = 0.10 mol/LEquilibrium constant (Keq) =?Equilibrium constant (Keq) = [NO]² / [N₂][H₂]
Equilibrium constant (Keq) = 0.1² / (0.5 × 0.3)
Equilibrium constant (Keq) = 0.01 / 0.15
Equilibrium constant (Keq) = 0.067
Thus, we can conclude that the equilibrium constant, Keq is 0.067
Learn more about equilibrium constant:
https://brainly.com/question/16589765
#SPJ1
What formula is used for solving problems involving Boyle’s law?P1T1=P2T2P1T1=P2T2P1=V2P1=V2V1T1=V2T2V1T1=V2T2P1V1=P2V2

Answers
Step 1 - Brief revision of Boyle's Law
Boyle's law is a law concerning the behavior of gases. It states that the pressure and the volume are inversely proportional given that the temperature is kept constant.
That is, if we increase the pressure, the volume will decrease. This principle is behind the proper functioning of our lungs, for example. This can also be described mathematically as:
\(P_1V_1=P_2V_2\)Step 2 - Choosing the right alternative
As we have seen in step 1, we can use the following formula to work with Boyle's Law:
\(P_1V_1=P_2V_2\)Therefore, the correct alternative is item d.
Use the following scenario to calculate the carbon flux in the atmosphere. The amount of carbon that enters the atmosphere through natural processes is 211.6 GtC/yr and the amount that leaves the atmosphere through natural processes in 213.8 GtC/yr. The amount of carbon released by burning fossil fuels is 5.5 GtC/yr. What is the carbon flux in the atmosphere
Answers
The amount of carbon flux in the atmosphere will be 3.3 GtC/yr
Carbon flux calculationThe carbon flux in the atmosphere can be calculated by:
1. Removing the amount of carbon that leaves the atmosphere from the amount that enters the atmosphere.
2. Adding the amount of carbon released by fossil fuel burning.
Thus,
Carbon flux = 211.6 - 213.8 + 5.5 = 3.3 GtC/yr
More on carbon flux can be found here: https://brainly.com/question/15857288
#SPJ12
what do you think will happen to the kidneys if any of these parts were damaged?
Answers
Answer:
When your kidneys are damage waste products and fluid can build up in your body. That can cause swelling in your ankles nausea weakness poor sleep and shortness of breath. so without treatement the damage can get worse and your kidney may eventually stop working.what particle decomposes to produce the electron of beta radiation
Answers
In beta radiation, the molecule that breaks down to create an electron could be a neutron or a proton
Beta radiation explained.
In beta radiation, the molecule that breaks down to create an electron could be a neutron or a proton. Beta radiation happens when a core experiences a change, coming about within the emanation of either a beta-minus molecule (electron) or a beta-plus molecule (positron).
In beta radiation a neutron inside the core changes over into a proton, emanating an electron and an antineutrino. The neutron is composed of quarks, particularly two down quarks and one up quark. Amid the rot, one of the down quarks inside the neutron changes into an up quark, changing the neutron into a proton. The overabundance vitality is carried absent by the transmitted electron (beta molecule) and the antineutrino.
The method can be spoken to by the taking after condition for beta-minus rot:
n → p + e- + ν-bar (antineutrino)
The abundance vitality is carried absent by the transmitted positron (beta molecule) and the neutrino.
The condition for beta-plus rot is as takes after:
p → n + e+ + ν (neutrino)
Learn more about beta radiation below.
https://brainly.com/question/16645044
#SPJ4
What causes a tea kettle that has been warmed on the stove to spew out water vapor
Answers
which of these molecules are ketones? check all that apply. a a three carbon chain. all carbons are bonded to h atoms. b a three carbon chain. one outer carbon is bonded to an o h group. c a three carbon chain. the middle carbon is double bonded to an o atom. d a three carbon chain. one outer carbon is double bonded to an o atom and single bonded to an h atom. e a three carbon chain. one outer carbon is double bonded to an o atom and single bonded to an o h group. f molecule h 3 c o c h 3.
Answers
Answer:
ldk
Explanation:
ldk
Z = 8, number of neutrons = 9 atomic symbol
Answers
According to Dalton's theory, is it possible to convert atoms of one element into atoms of another? Explain.
please help
Answers
Answer:
No, it's not possible to change the atoms into a different element.
Explanation:
No, because rule four of his theory says that change cannot occur
If a driver takes 30 min to get to the town market, which is 1.5 km away from his house, what is his average speed?
Note: Unit for average speed is km/h
Answers
Answer: 0.83 m/s
Linear motion consists of 2: constant velocity motion with constant velocity and uniformly accelerated motion with constant acceleration
Turn 30min to seconds (divided by 60)
1.5km/h: 0.5s =3km/h
______ occurs when aqueous solutions of ammonia and vinegar are mixed
Answers
Formation of ammonium acetate and water occurs when aqueous solutions of ammonia and vinegar are mixed, the
Ammonia, which has the chemical formula NH₃, is a weak base, while vinegar, which is a solution of acetic acid (CH₃COOH) in water, is an acid. When the two solutions are mixed, a neutralization reaction occurs, in which the ammonium ion (NH⁴⁺) from ammonia combines with the acetate ion (C₂H₃O²⁻) from vinegar to form ammonium acetate (NH₄C₂H₃O₂) and water (H₂O).
The balanced chemical equation for the reaction is,
NH₃(aq) + CH₃COOH(aq) → NH₄C₂H₃O₂(aq) + H₂O(l)
To know more about the ammonia, here
brainly.com/question/15409518
#SPJ4
What mass of chromium would be produced from the reaction of 57.0 g of potassium with 199 g of chromium(II) bromide according to the following reaction? 2 K + CrBr2 2 KBr + Cr
Answers
Answer:
Mass of Chromium produced = 37.91 grams
Explanation:
2K + CrBr₂ → 2KBr + Cr
2mole 1 mole 1 mole
mass of Potassium = 57.0 grams
molar mass of Potassium = 39.1 g/mol
no of moles of Potassium = 57.0 / 39.1 = 1.458 moles
mass of CrBr₂= 199 grams
molar mass of CrBr₂ = 211.8 gram/mole
no of moles of CrBr₂ = 199 / 211.8 = 0.939 mole
From chemical equation
1 mole of CrBr₂ = 2 moles of K
∴ 0.939 moles of CrBr₂ = ?
⇒ 0.939 x 2/1 = 1.878 moles of K
1.878 moles of K is needed, but there is 1.458 moles of K. So, Potassium is completed first during the reaction . Hence, Potassium is limiting reagent. and CrBr₂ is excess reagent .
From chemical equation
2 moles of K = 1 mole of Cr
∴ 1.458 moles of K = ?
⇒ 1.458 x 1/ 2 = 0.729 moles of Cr
no of moles of Cr formed = 0.729 moles
molar mass of Cr = 52.0 g/mol
mass of one mole of Cr = 52.0 grams
mass of 0.729 moles of Cr = 52.0 x 0.729 = 37.908 grams
mass of Chromium produced = 37.91 grams
crystallization of sugar in a syrup can be avoided by addition of
Answers
Crystallization of sugar in a syrup can be avoided by adding inverted sugar. Invert sugar is a mixture of glucose and fructose, produced from sucrose. It is produced by hydrolysis of sucrose by heating with water and an acid such as lemon juice, vinegar or cream of tartar.
By adding invert sugar to a syrup, it reduces the amount of sucrose present in the solution and promotes the formation of glucose and fructose. This causes the solution to become more saturated and the syrup less likely to crystallize. Invert sugar is often used in the production of confectionery, ice cream, and other sweets.Inverted sugar can also be used to improve the texture and quality of baked goods. It is often used in cake recipes to enhance the flavor and increase the shelf life. It can also be used as a substitute for honey or corn syrup in recipes that require a liquid sweetener.In conclusion, the addition of invert sugar helps to prevent crystallization of sugar in syrup. Invert sugar is a mixture of glucose and fructose produced from sucrose by hydrolysis.
To know more about Crystallization visit :
brainly.com/question/32130991
#SPJ11
why was the discovery of high temperature superconductors so startling to scientists?
Answers
It is because the high temperature superconductor was more affordable، easy to use and more efficient than before.
Hope it helps you...
Please mark brainliest if it helps you.
Answered by Benjemin
Can someone please help and list what each type of thing they are? (Element, Compound, Mixture of elements, Mixture of compounds, Mixture of elements and compounds)

Answers
2. C
3. E
4. D
5. A
6. B
7. A
8. E
9. A
Hope this helps!
Sometimes when performing a crystallization, one solvent alone will not work and you have to use a solvent-pair. Will the solvent pair hexane and diethyl ether work? why or why not?.
Answers
Yes, hexane and diethyl ether, a solvent pair, will definitely function as a team during crystallization.
This is due to the fact that crystal formation requires a miscible solvent pair. Normally, two solvents—one in which the compound is soluble and one in which it is not—are required for crystallization. Additionally, these two solvents ought to mix easily.
Hexane and diethyl ether, the given solvent pair, are miscible with one another. Therefore, we can crystallize using the solvent pair mentioned above.
What is crystallization?
Crystallization is the process of increasing the concentration of a solution to a supersaturated state in order to form solid particles in a homogeneous phase. Crystallization is the process by which crystals are formed from either melted material or a solution.
Find more on Crystallization at : brainly.com/question/4155452
#SPJ4
Erica is working in the lab. She wants to remove the fine dust particles suspended in a sample of oil. Which method is most likely to yes? A reverse osmosis b osmosis c filtration d dilution
Answers
Answer: reverse osmosis.
Explanation:
you are welocome please choose me for brainlist
A weather balloon is designed to burst when the volume reaches 250.0 L. The balloon is filled with 71.6 L helium at sea level where the pressure is 1.00 atm at 20.0 °C. The balloon expands and bursts after ascending until the temperature is -35.0 °C. Determine the pressure (in psi) at which the balloon bursts.
Answers
7.33 psi is the pressure at which the balloon bursts.
What are the weather balloon's pressure and volume?With a pressure of 748 mmHg and a temperature of 28.0 °C, a weather balloon is inflated to a volume of 28.5 L. The balloon ascends through the atmosphere to a height of around 25,000 feet, where the temperature is -15.0 °C and there is a pressure of 385 mmHg. Release of the balloon into the atmosphere. The pressure on the balloon is 35.0 kPa at the height above the ground.
P1 vs. V1 = P2 vs. V2 vs. T2 (T2)
Where:
P1 = 1.00 atm
V1 = 71.6 L
T1 = 293.15 K (20.0 °C in Kelvin)
T2 = 238.15 K (-35.0 °C in Kelvin)
V2 = 250.0 L
P2 = (P1 V1 T2) / (V2 T1) (V2 T1)
P2 = (1.00 atm * 71.6 L * 238.15 K) / (250.0 L * 293.15 K)
P2 = 0.499 atm
1 atm = 14.696 psi
0.499 atm = (0.499 atm) * (14.696 psi / 1 atm) = 7.33 psi
Hence, 7.33 psi is the pressure at which the balloon bursts.
To know more about pressure visit:-
https://brainly.com/question/19484297
#SPJ1
What is mass in grams of 2.30 moles of Aluminum?
Answers
62.1 g Al
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableStoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
2.30 mol Al
Step 2: Identify Conversions
[PT] Molar Mass of Al - 26.98 g/mol
Step 3: Convert
Set up: \(\displaystyle 2.30 \ mol \ Al(\frac{26.98 \ g \ Al}{1 \ mol \ Al})\)Multiply/Divide: \(\displaystyle 62.054 \ g \ Al\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
62.054 g Al ≈ 62.1 g Al
what could you do to increase the electrical force between two charged particles by a factor of 16
Answers
Increasing one charged particle by 16 will lead to increasing the electrical force between two charged particles by a factor of 16.
What is Electrical force?This is referred the attractive or repulsive interaction between any two charged bodies present.
F= kq1q2/r²
F1/F2= kq1q2/r² / 16kq1q2/r²
F1/F2 = 1/16
F2 = 16F1
This is therefore how to increase the electrical force between two charged particles by a factor of 16.
Read more about Electrical force here https://brainly.com/question/1498868
#SPJ1
Calculate the frequency of 4.1 x 10^-19
Answers
Complete the ion symbol for the
atom below.

Answers
Answer:Helium
Explanation:
Helium can hold two electrons on the outer shell. as for the inner part it mostly looks like helium.
How many neutrons does a titanium have if its mass number is 48 and electrons are 22?
Answers
If I have 9.7 moles of gas at a pressure of 0.12 atm and at a temperature of 46°C, what is the volume of the
container that the gas is in?
Answers
Answer:
2117.02 litres
Explanation:
Using the ideal gas equation as follows;
PV = nRT
Where:
P = pressure of the gas (atm)
V = volume of the gas (L)
n = number of moles (mol)
R = gas law constant (Latm/molK)
T = temperature (K)
According to the information given in this question,
P = 0.12 atm
V = ?
n = 9.7moles
T = 46°C = 46 + 273 = 319K
R = 0.0821 Latm/molK
Using PV = nRT
0.12 × V = 9.7 × 0.0821 × 319
0.12V = 254.04
V = 254.04 ÷ 0.12
V = 2117.02 litres
what is the mass of water which can dissolve 8.6 g of lithium chloride in a 6.70% by mass solution
Answers
Answer:
~120 grams water
Explanation:
% LiCl = (Mass LiCl) / (Mass of Soln) x 100%
= Mass LiCl / (Mass H₂O + Mass LiCl) x 100% = 6.7% (divide both sides by 100%)
=> Mass LiCl / Mass H₂O + Mass LiCl = 0.067
=> Mass LiCl = 0.067(Mass H₂O) + 0.067(Mass LiCl)
Solve for Mass H₂O:
=> Mass LiCl - 0.067(Mass LiCl) = 0.067(Mass H₂O)
=> Mass H₂O = (Mass LiCl - 0.067(Mass LiCl) / 0.067
= 8.6g - 0.067(8.6g) / 0.067 = 8.0238g / 0.067 = 119.76g H₂O = ~120grams
give the systematic, or iupac, name for the compound. a six carbon ring is bonded to a four carbon ring.
Answers
The systematic, or IUPAC, name for the compound in which a six-carbon ring is bonded to a four-carbon ring is cyclohexylbutylcyclobutane.
To name the compound given the chemical structure -Naming an organic compound follows a set of rules. These rules are widely known as nomenclature, which gives a unique name to a compound.
In this compound, there is a six-carbon ring, which is cyclohexyl and a four-carbon ring, which is cyclobutane. Both are linked by a butyl group. We can apply the following rules to name this compound:Identify the parent chain: In this case, it is the ring with the maximum number of carbons, which is cyclohexyl. Remove the suffix -ane from the parent chain: Cyclohexyl. Remove the name of the side chain that is bonded to the parent chain: Butyl. Add the name of the smaller ring with its prefix: cyclobutane. Finally, the correct name of the compound is Cyclohexylbutylcyclobutane.
To know more about IUPAC visit:
https://brainly.com/question/30086566
#SPJ11