Answers
Answer:
It’s is a simple
Explanation:the prokaryotic cell involves a simple process which intern involves one chromosom
Related Questions
Calcium reacts with hydrochloric acid to produce calcium chloride and hydrogen gas:
19
Ca (s) + 2 HCI (1) - CaCI2 (aq) + H2 (g)
When 1.911 g of Ca is combined with enough hydrochloric acid to make 200.0 mL of solution in a coffee-cup calorimeter, all of the Ca reacts, raising the temperature of the solution from 26.2 °C to 29.2 °C. Find the AHrn in kJ for the reaction as written. (Assume that the specific heat capacity of the solution is 4.184 J/g °C and the density is 1.00 g/mL.)
Answers
The enthalpy change of the reaction is 52.6604 kJ/mol.
What is Enthalpy?
Enthalpy is a thermodynamic property that describes the amount of heat released or absorbed by a system at constant pressure. It is represented by the symbol H and is measured in units of joules (J) or kilojoules (kJ).
To find the enthalpy change of the reaction (ΔH), we can use the formula:
ΔH = q / n
where q is the heat absorbed or released by the reaction, and n is the number of moles of Ca that reacted.
First, we need to calculate the heat absorbed by the solution. We can use the formula:
q = m × c × ΔT
where m is the mass of the solution (in grams), c is the specific heat capacity of the solution (in J/g°C), and ΔT is the change in temperature (in °C).
We can calculate the mass of the solution using its density:
mass = volume × density
mass = 200.0 mL × 1.00 g/mL
mass = 200.0 g
The change in temperature is:
ΔT = 29.2 °C - 26.2 °C
ΔT = 3.0 °C
Now we can calculate q:
q = 200.0 g × 4.184 J/g°C × 3.0 °C
q = 2510.4 J
Next, we need to calculate the number of moles of Ca that reacted. We can use the mass of Ca and its molar mass:
n = m / M
The molar mass of Ca is 40.08 g/mol.
n = 1.911 g / 40.08 g/mol
n = 0.0476 mol
Finally, we can calculate the enthalpy change of the reaction:
ΔH = q / n
ΔH = 2510.4 J / 0.0476 mol
ΔH = 52660.4 J/mol
Converting to kJ/mol:
ΔH = 52.6604 kJ/mol
Learn more about Enthalpy from the given link
https://brainly.com/question/30431725
#SPJ1
Ignoring the CuSO4, and based solely on the volume and molarity of the Na2S(aq), how many grams of CuS(s) would be produced by this
Answers
Answer:
0.019122 grams of CuS(s) would be produced .
Explanation:
Given , volume of \(CuSO_{4}\) is 4ml = 0.004 L
volume of \(Na_{2}S\) is 2ml = 0.002 L
\(CuSO_{4} + Na_{2}S $\rightarrow$ Na_{2}SO_{4} + CuS\)
Moles of \(CuSO_{4}\) = Molarity x volume (L)
= 0.1M x 0.004 L
= 0.0004 moles
Moles of \(Na_{2}S\) = 0.1M x 0.002 L
= 0.0002 moles
1 mole of \(Na_{2}S$\rightarrow$\) 1 mole of \(CuS\)
Therefore , 0.0002 moles \(Na_{2}S $\rightarrow$\) 1mole of \(CuS\)
Mass of \(CuS\) = Moles x molar mass
= 0.0002 x 95.611
= 0.019122 grams .
Hence the answer is 0.019122 grams.
Cell Membrane Transport
List 5 observations when you open the blue and green gated channels:
Section 2
After listing your observation, click “reset all” and do the same process again, following the instructions listed above. Answer the following questions below.
1.) Open the green gated channel and observe. What did you observe when you open the green gated channel?
2.) Based on your observations, what type of solution have you observed? Is it ‘hypertonic’, ‘hypotonic’ or ‘isotonic’? Explain
3.) Open the blue gated channel. Observe on what will happen when you open the blue gated channel. What have you observed?
4.) What type of solution have you observed? Is it ‘hypertonic’, ‘hypotonic’ or ‘isotonic’? Explain.
5.) Slow down the animation. What did you observed on the motion of the green circles and blue diamonds?
Answers
A type of fossil fuel,known as Tar stands.
what is fossil fuel?A fossil fuel is a hydrocarbon-containing material formed naturally in to the Earth's crust from to the remains of dead plants and animals and birds that is the extracted and burned as thr a fuel. The main fossil fuels are mainly coal, oil, and natural gas.
Tar sands are a combination of clay, sand, water and bitumen, which is a heavy hydrocarbon. Like the kerogen in oil shale, tar sands' bitumen can be upgraded to synthetic crude oil.
So answer is tar sands.
These include tar sands – deposits of moist sand and it is clay with 1-2 percent bitumen (thick and heavy are petroleum rich in the carbon and poor in the hydrogen). These are the removed by strip mining in the (see section below on coal)
To know more about fossile fuel click-
https://brainly.com/question/79954
#SPJ1
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
Select the statements that correctly describe buffers.
A) A buffer is made up of a strong acid and a strong base.
B) The pH of a buffer solution does not change significantly when a small amount of acid is added.
C) The pH of a buffer solution is determined by the ratio of the concentration of acid to the concentration of base.
D) The K_a of a buffer decreases slightly when a small amount of acid is added to the buffer solution.
E) A strong acid added to the buffer solution reacts with the weak acid of the buffer.
Answers
Answer:
B) The pH of a buffer solution does not change significantly when a small amount of acid is added.
C) The pH of a buffer solution is determined by the ratio of the concentration of acid to the concentration of base.
Explanation:
Buffer solution is useful in maintaining the pH of a solution constant . It is made of a weak acid and its salt or a weak base and its salt . Ph of a buffer is given by the following relation .
pH = pKa + log [ A⁻ ] / [ HA ]
A- is a base and HA is a weak acid .
When we add acid , it reacts with A⁻ or base and gets neutralised .
When we add base , it reacts with acid HA and gets neutralised . In this way it maintains the pH of the solution .
Correct statements are as follows:
B) When a tiny amount of solvent is introduced to a buffer, the pH doesn't really alter considerably.
C) The ratio of acids to base concentration determines the pH of a phosphate buffer.
So, options B) and C) are correct.
Define pH.ph scale used in science to measure the acidity or alkalinity of an aqueous solution. pH originally stood for "potential of hydrogen." The pH of acidic solutions is lower than that of basic or alkaline solutions.
Correct statements are as follows:
B) When a tiny amount of solvent is introduced to a buffer, the pH doesn't really alter considerably.
C) The ratio of acids to base concentration determines the pH of a phosphate buffer.
So, options B) and C) are correct.
Find out more information about pH here:
https://brainly.com/question/15289741?referrer=searchResults
Describe an element in two examples
Answers
Answer:
Explanation:
An element is the simplest form of matter that has a unique set of properties. Examples of well-known elements include oxygen, iron, and gold (see the figure below). Elements cannot be broken down into simpler substances. Likewise, one element cannot be chemically converted into a different element.
Explanation:
An element is a pure substance that is made from a single type of atom. Elements are the building blocks for all the rest of the matter in the world. Examples of elements include iron, oxygen, hydrogen, gold, and helium. An important number in an element is the atomic number.
Not sure if it's your asking in science
What is the part of the neuron that receives information from other neurons,
muscles, or glands
Answers
At 25∘C, the Henry's law constant for CO2 is 0.034Matm. What pressure of carbon dioxide is needed to maintain a CO2 concentration of 0.80 M?
Answers
Answer:
P = (Henry's law constant) * (CO2 concentration)
P = (0.034 M/atm) * (0.80 M) = 0.027 atm.
In order to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius, a pressure of about 23.53 atm would be needed according to Henry's Law.
In the field of Chemistry, Henry's law is used to describe the solubility of gases in liquids. This law states that at a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid.
Using the given values from the problem, where the Henry's Law constant (KH) is 0.034 M⋅atm and the desired CO_2 concentration (C) is 0.80 M, we can insert these into the formula for Henry's law: P = C/KH.
By substituting the relevant values, you get P = 0.80 M / 0.034 M⋅atm which equates to approximately 23.53 atm. Therefore, a pressure of about 23.53 atm of carbon dioxide would be needed to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius.
Learn more about Henry's Law here:
https://brainly.com/question/35540348
#SPJ2
A 250 mL sample of gas is collected over water at 35°C and at a total pressure of 735 mm Hg. If the vapor pressure of water at 35°C is 42.2 torr, what is the volume of the gas sample at standard pressure?
Answers
Answer:
The volume of the gas sample at standard pressure is 819.5ml
Explanation:
Solution Given:
let volume be V and temperature be T and pressure be P.
\( V_1=250ml\)
\( V_2=?\)
\( P_{total}=735 mmhg\)
1 torr= 1 mmhg
42.2 torr=42.2 mmhg
so,
\( P_{water}=42.2mmhg\)
\( T_1=35°C=35+273=308 K\)
Now
firstly we need to find the pressure due to gas along by subtracting the vapor pressure of water.
\( P_{gas}=P_{total}-P_{water} \)
=735-42.2=692.8 mmhg
Now
By using combined gas law equation:
\(\frac{P_1*V_1}{T_1} =\frac{P_2*V_2}{T_2}\)
\(V_2=\frac{P_1*}{P_2}*\frac{T_2}{T_1} *V_1\)
\(V_2=\frac{P_gas}{P_2}*\frac{T_2}{T_1} *V_1\)
Here \(P_2 \:and\: T_2\) are standard pressure and temperature respectively.
we have
\(P_2=750mmhg \:and\: T_2=273K\)
Substituting value, we get
\(V_2=\frac{692.8}{750}*\frac{273}{308} *250\)
\(V_2= 819.51 ml\)
As you move down a group the electronegativity of an element will
Answers
matrix composites of this type are noted for improving the resistance to fracture of their dispersive material.
Answers
This kind of matrix composite is renowned for strengthening the dispersive material's resistance to fracture. metals, ceramics, and polymers.
Ceramic matrix composites (CMC) are substances made of both ceramic and composite components.It consists of two parts: a matrix that holds the material together and a reinforcement that gives the material its tensile strength. When ceramic matrix composites have a scattered phase, it is primarily because the material's toughness has increased, which has increased the tensile strength.
To learn more about Ceramic matrix composites :
https://brainly.com/question/12974524
#SPJ4
Imagine that the reaction in model 1 starts with 100 molecules of A and zero molecules of B. explain why the concentration of substance A will never reach zero.
Answers
What type of bonding does Ir and Hg have?
Answers
Iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
Iridium (Ir) and mercury (Hg) exhibit different types of bonding based on their electronic configurations and properties.
Iridium is a transition metal belonging to Group 9 of the periodic table. It has a partially filled d-orbital in its atomic structure, which allows it to form metallic bonds. Metallic bonding occurs when the outer electrons of metal atoms are delocalized and form a "sea" of electrons that are free to move throughout the crystal lattice. This results in the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility. Iridium forms metallic bonds with other iridium atoms, contributing to its solid, dense, and lustrous nature.
Mercury, on the other hand, is a unique element. It is a transition metal, but it exhibits characteristics of both metallic and covalent bonding. At room temperature, mercury exists as a liquid, which is highly unusual for a metal. This is because mercury atoms have a weak interatomic interaction, known as metallic bonding, similar to other metals. However, due to the presence of unpaired electrons in its 6s orbital, mercury can also form weak covalent bonds. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
In summary, iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding.
For more question on bonds
https://brainly.com/question/29794367
#SPJ8
Based on Robert's knowledge of physical properties of matter, describe how he can do this?
Answers
Matter of the substance is the mass and takes up space, volume . .The physical properties of matter is the that determine without changing chemical identity.
The properties of matter is of two types : the physical property and the chemical property . A physical properties of matter is the property that can be determined without changing their chemical identity of a substance. example : density , color, mass , volume , length, etc. the physical properties are of two types : intensive physical properties and the extensive physical properties.
The chemical properties of that that can be determine by the changing the chemical identity of substance. example : toxicity, heat, enthalpy, flammability.
Thus, Matter of the substance is the mass and takes up space, volume .The physical properties of matter is the that determine without changing chemical identity.
To learn more about physical properties here
https://brainly.com/question/19532631
#SPJ1
Which compound is insoluble in water?

Answers
Answer:
The answer is C... I am almost positive.
What affects how sound travels? (Select all that apply.)
a pressure
b temperature
c wavelength
d medium
Answers
Answer:
A. pressure and C. wavelength
Explanation:
All sound is different and many are affected differently but pressure and wavelength of it are the main ones.
what’s is the answer?

Answers
The energy of the photon of light can be obtained as 6.27 * 10^-20 J.
What is the energy of the photon?We know that a photon has to to do with a particular unit of light. We know that light can be said to be composed of very tiny corpuscles and these corpuscles of light is what we call the photon of the light.
We can be able to us the equation that is derived by Max Plank to be able to get the value of the energy of the photon of light. Now we know that a photon of light can have an energy that is able to be obtained by;
E = hf
h = Plank's constant
f = Frequency
Then;
E = 6.6 * 10^-34 Js * 9.5 * 10^13 Hz
= 6.27 * 10^-20 J
Thus as we can see from the parameters in the question, the energy of the photon is 6.27 * 10^-20 J.
Learn more about energy of the photon:https://brainly.com/question/2393994
#SPJ1
Solids have a______ shape and ________ volume. *
O Defined shape and undefined volume
O Undefined shape and undefined volume
O Defined shape and defined volume
O Undefined shape and defined volume
Answers
Solids have a Defined shape and defined volume. A solid has an exact quantity and shape, a liquid has a definite extent but no exact shape and gasoline has neither a particular volume nor shape.
Volume is the measure of the potential that an object holds. for instance, if a cup can maintain 100 ml of water as much as the brim, its volume is stated to be one hundred ml. extent can also be defined as the quantity of space occupied by a three-dimensional item.
Volume is the degree of the 3-dimensional area occupied by means of matter or enclosed by a surface, measured in cubic gadgets. The SI unit of quantity is the cubic meter (m3), which is a derived unit. Liter (L) is a unique call for the cubic decimeter (dm3).
Special approaches to discovering volume:-
clear up for quantity through space. All physical items occupy the area, and you could discover the volume for a number of them by using measuring their bodily dimensions. solve for quantity with the aid of Density and Mass. Density is defined as an object's mass in line with a given unit of quantity. ...resolve for extent by using Displacement.Learn more about volume here:
brainly.com/question/1972490
#SPJ9
What volume of 12 gas forms when 21 L Cl2 react at STP?
Answers
The volume of I₂ gas formed when 21 L of Cl₂ gas reacts at STP is 21 L
How do I determine the volume of I₂ gas formed?We'll begin by writing the balanced equation for the reaction. This is shown below:
2KI(aq) + Cl₂(g) -> 2KCl(aq) + I₂ (g)
From the balanced equation above,
1 L of Cl₂ reacted to produce 1 L of I₂
With the above information, we can obtain the volume of I₂ formed when 21 L of Cl₂ react at STP as follow: :
From the balanced equation above,
1 L of Cl₂ reacted to produce 1 L of I₂
Therefore
21 L of Cl₂ will also react to produce 21 L of I₂
Thus, the volume of I₂ formed is 21 L
Learn more about volume:
https://brainly.com/question/9614052
#SPJ1
A teacher uses beans to model a chemical reaction. The model is shown in the diagram.
Model of a chemical reaction shown by using beans. Three beans represented as solid reacts with three beans represented as liquid produces three beans of solid and three beans of gas.
According to the law of conservation of matter, how does the combined mass of C and D compare to the combined mass of A and B?
A. The combined mass of C and D is slightly less than the combined mass of A and B.
B. The combined mass of C and D is the same as the combined mass of A and B.
C. The combined mass of C and D is slightly more than the combined mass of A and B.
D. The combined mass of C and D is very different from the combined mass of A and B.
Answers
Answer:B
Explanation:
Law of conservation of mass states how many you start with is how many you end with. So you started with 6 beans (3 of each) and you end with 6 beans.
Are all the molecules in the picture the same?
Answers
Answer:
There is no picture.
Answer:
yes because they are together yp diffrent moleucoles
Explanation:
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
what product is formed when Manganese (iv) Chloride reacts with Cobalt (ii)Nitrate
Answers
Answer:
Manganese(iv)Nitrate and Copper(ii)Chloride
Something that changes matter is called
Answers
Answer:
chemical changes or chemical reactions
2) What mass of iron starting at 100.0 oC must be added to 50.0 g of water at 25.0 oC to increase the water’s temperature to 65.0 oC? Iron’s specific heat capacity is 0.450 J/(g oC).
Answers
Taking into account the definition of calorimetry, the mass of iron that must be added is 531.556 grams.
CalorimetryCalorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
When heat added or removed from a substance causes a temperature change in it without affecting its molecular structure, it is called sensible heat.
So, the equation that allows to calculate heat exchanges is:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
Mass of ironIn this case, you know:
For iron:Mass of iron= ?Initial temperature of iron= 100 °CFinal temperature of iron= 65 ºCSpecific heat of iron = 0.450 J/(g°C)For water:Mass of water = 50 gInitial temperature of water= 25 ºCFinal temperature of water= 65 ºCSpecific heat of water = 4.186 J/(g°C)Replacing in the expression to calculate heat exchanges:
For iron: Qiron= 0.450 J/(g°C) × mass of iron× (65 °C - 100 °C)
For water: Qwater= 4.186 J/(g°C) × 50 g× (65 °C - 25 °C)
If two isolated bodies or systems exchange energy in the form of heat, the quantity received by one of them is equal to the quantity transferred by the other body. That is, the total energy exchanged remains constant, it is conserved.
Then, the heat that the gold gives up will be equal to the heat that the water receives. Therefore:
- Qiron = + Qwater
- 0.450 J/(g°C) × mass of iron× (65 °C - 100 °C)= 4.186 J/(g°C) × 50 g× (65 °C - 25 °C)
Solving:
15.75 J/g × mass of iron= 8372 J
mass of iron= 8372 J÷ 15.75 J/g
mass of iron= 531.556 grams
Finally, the mass of iron necessary is 531.556 grams.
Learn more about calorimetry:
brainly.com/question/11586486
brainly.com/question/24724338
brainly.com/question/14057615
brainly.com/question/24988785
#SPJ1
what is the relative rate of diffusion of hydrogen and nitrogen
Answers
Answer:
2.645
Explanation:
Rate of diffusion formula:
Sqrt(mass2/mass1)
>>sqrt(14/2)
(Note:Hydrogen must exist in dwiatomic, [H2])
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
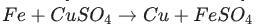
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
For equal masses the
substance occupies the smaller volume.
Answers
For equal masses of a set of substances, the substance with the lightest unit mass will occupy the most volume.
his is further explained below.
What is mass?Generally, A physical body's mass may be defined as the amount of matter that it contains. Additionally, it is a measurement of the body's inertia, which is the resistance to acceleration when a net force is applied to the system.
A volume is a measurement of the amount of space that is occupied in three dimensions.
In conclusion, When all of the individual masses of a group of substances are the same, the one that has the lowest unit mass will take up the most space.
Read more about mass
https://brainly.com/question/19694949
#SPJ1
The substance occupies a smaller volume
In equivalent masses, the substance occupies smaller volume?Mass is the quantity of matter an object contains, while the volume is much space take a hold of up. Example: A bowling ball and a basketball are much the same volumes as each other, but the bowling ball has substantially more mass. Volume is the quantity of area occupied by a substance, while mass is the amount of matter it carry. The amount of mass per unit of volume is a sample's substance. Volume is the size of the capacity that an object holds. For example, if a cup can hold 100 ml of water up to the peak, its volume is said to be 100 ml. Volume can also be defined as the amount of space occupied by the 3-dimensional object.
so we can conclude the substance occupies a smaller volume,
Learn about more here: masses https://brainly.com/question/28694445
#SPJ1
2 NaClO3 → 2 NaCl + 3 O2
Calculate the mass of O2 produced as the result of the decomposition of 843 g of NaClO3.
Answers
Taking into account the reaction stoichiometry, 380.12 grams of O₂ are produced as the result of the decomposition of 843 g of NaClO₃.
Reaction stoichiometryIn first place, the balanced reaction iS:
2 NaClO₃ → 2 NaCl + 3 O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
NaClO₃: 2 molesNaCl: 2 molesO₂: 3 molesThe molar mass of the compounds is:
NaClO₃: 106.45 g/moleNaCl: 58.45 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
NaClO₃: 2 moles ×106.45 g/mole= 212.9 gramsNaCl: 2 moles ×58.45 g/mole= 116.9 gramsO₂: 3 moles ×32 g/mole= 96 gramsMass of O₂ formedThe following rule of three can be applied: if by reaction stoichiometry 212.9 grams of NaClO₃ form 96 grams of O₂, 843 grams of NaClO₃ form how much mass of O₂?
mass of O₂= (843 grams of NaClO₃× 96 grams of O₂) ÷212.9 grams of NaClO₃
mass of O₂= 380.12 grams
Finally, 380.12 grams of O₂ are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1