Predict which substance has greater molar entropy. Explain.(c) Na(s) or K(s)
Answers
K (Potassium) has higher molar entropy than sodium.
The substance's condition has the most impact on the molar entropy. Compounds that are gases will have a far higher molar entropy than compounds that are liquids or solids because gases are much more widely dispersed. A substance's entropy rises as its molecular weight, complexity, and temperature rise. As the pressure or concentration decreases, the entropy likewise rises. Gas entropies are significantly higher than those of condensed phases. The highest entropy is in gases. This is due to the wide variety of microstates in which gases can reside. The amount of freedom that atoms in a substance have to disperse, migrate, and organize themselves randomly is referred to as entropy.
Learn more about molar entropy here-
https://brainly.com/question/17176334
#SPJ4
Related Questions
Krypton has six stable isotopes. How many neutrons are in the most abundant isotope of krypton according to the mass spectrum in Figure P2.10? Write the AX form of the symbol of that isotope.
Answers
The number of neutrons in the most abundant isotope of krypton according to the mass spectrum is 48.
What are neutrons?The neutron is a subatomic particle with the sign n or n0, a neutral charge, and slightly more mass than a proton. Atoms' nuclei are made up of protons and neutrons. Except for basic hydrogen, every atom's nucleus contains a neutron, a subatomic particle. The name of the particle comes from the fact that it is electrically neutral and devoid of charge. The densest particles are neutrons.
A category of a subatomic particle having a positive charge is the proton. The strong nuclear force holds the protons together in the atom's nucleus. A subatomic particle with no charge is called a neutron (they are neutral)
You can find out how many protons, neutrons, and Z an isotope has in its nucleus by looking at its mass number A. Z is determined by the atomic number.
A = Z + number of atoms
krypton-84 So
A = 84
Number of neutrons
A - Z
= 48
To know more about neutrons, visit:
https://brainly.com/question/18501839
#SPJ9
Ion channels in the axon membrane open and close based on __________, whereas ion channels in the dendrite membranes open and close based on ___________.
Answers
Ion channels in the axon membrane open and close based on changes in the membrane potential, whereas ion channels in the dendrite membranes open and close based on neurotransmitter binding.
In the axon membrane, ion channels are sensitive to changes in the electrical potential across the membrane. These channels, known as voltage-gated ion channels, respond to specific thresholds of depolarization or hyperpolarization. When the membrane potential reaches a certain threshold, these channels open, allowing the flow of ions such as sodium, potassium, and calcium. This process is crucial for generating and propagating action potentials along the axon.
On the other hand, ion channels in the dendrite membranes are primarily influenced by neurotransmitter binding. Dendrites receive signals from other neurons through synapses. When a neurotransmitter is released from the presynaptic neuron and binds to the receptors on the dendrite, it initiates a series of biochemical events that lead to the opening or closing of ion channels. These channels, known as ligand-gated ion channels or ionotropic receptors, are specific to the neurotransmitter they interact with. The opening of these channels allows ions to flow across the dendrite membrane, generating local potentials or synaptic potentials.
In summary, ion channels in the axon membrane open and close based on changes in the membrane potential, whereas ion channels in the dendrite membranes open and close based on neurotransmitter binding. These distinct mechanisms contribute to the overall function of neurons in receiving and transmitting electrical signals.
Learn more about Ion channels here:-
https://brainly.com/question/30784325
#SPJ11
If I initially have a gas at a pressure of 1260 kPa, a volume of 23 liters, and a temperature of 200 K, and
then I raise the pressure to 1595 kPa and increase the temperature to 300 K, what is the new volume of
the gas?
Answers
______ = ________
T1 T2
1260 x 23 / 200 = 144.9
1595x V/300
144.9= 1595x V/300
144.9 x 300/1595 = v
27.25 litres
I think
Explain why all glassware must be dry and the solvent anhydrous during formation and reaction of a Grignard reaction. Explain why it is advisable to not clean your glassware with acetone before a Grignard reaction. Include balanced chemical equations in your answer. (4 pts)
Answers
Answer:
The Grignard reagent will be destroyed
Explanation:
The Grignard reaction is based on a carbanion production (RMgX). If water is present in the glassware, a reaction can take place with \(H_2O\) and the desired product will not be produced. Because an "H" would be transferred from water to the Grignard reagent. (See figure 1)
If we have acetone in the glassware we will have a similar problem. The Grignard reagent would be destroyed and the desired product will not be produced. The Grignard reagent will attack the carbon in the carbonyl group and the carbanion would disappear. (See figure 2)


when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. calculate ka for hx. report your answer rounded to two significant figures using e- notation.
Answers
when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. ka for hx is 3.405 × \(10^{-5}\)
Number of moles = 0.367
Volume of solution = 2l
concentration = 0.367/2 = 0.1835 mol/L
ph = 2.60
we know
ph = - log [H+]
2.51 × \(10^{-2.60}\)M = [H+]
The acid HX dissociate as
HX → H+ + X-
The acid dissociation constant Ka, for the dissociation reaction is
Ka = [H+][X-]/[HX] ; at equilibrium, [H+] = [X-]
Ka = 3.405 × \(10^{-5}\)
A solution in which water serves as the solvent is called an aqueous solution. The most common way to represent it in chemical equations is to add (aq) to the appropriate chemical formula. For instance, the formula for a solution of table salt, or sodium chloride (NaCl), in water is Na+(aq) + Cl The word aqueous, which derives from the word aqua, means that something is connected to, resembles, or is dissolved in water. Water is a common solvent in chemistry because it is an excellent solvent and abundant in nature. Since water is frequently used as the experiment's solvent, unless otherwise stated, the term "solution" refers to an aqueous solution.
Learn more about Aqueous solution here:
https://brainly.com/question/14097392
#SPJ4
Please help!!!
Multi-Step Problems
1. How many liters would be taken up by 2.50 g of helium?
2. How many grams are in 5.0 x 10^25atoms of sodium?
3. How many atoms are in 3.95 grams of Ni?
4. How many atoms are in a 15 kg sample of XE gas?
5. How many liters would be taken up by 45,000,000 atoms of Ar?
6. How many atoms are in a 75g sample of CO₂?

Answers
2.50 g of helium gas at STP would occupy a volume of 14.0 liters.
What is STP?Standard temperature and pressure (STP) conditions of 0°C and 1 atm, we can use the molar volume of a gas at STP, which is 22.4 L/mol.
1. Helium seems to have a molar mass of 4.00 g/mol.
Therefore, we can calculate the number of moles of helium as:
moles of He = mass of He divided by molar mass of He
= 2.50 g / 4.00 g/mol
= 0.625 mol
Then, we can use the molar volume of a gas at STP to calculate the volume of helium gas:
volume of He = moles of He x molar volume of gas at STP
= 0.625 mol x 22.4 L/mol
= 14.0 L
2.50 g of helium gas at STP would occupy a volume of 14.0 liters.
2. The molar mass of sodium is 22.99 g/mol. Therefore, we can calculate the number of moles of sodium as:
moles of Na = number of atoms of Na divided by Avogadro's number
= 5.0 x 10²⁵ atoms / 6.022 x 10²³ atoms/mol
= 83.0 mol
Then, we can use the molar mass of sodium to calculate the mass of sodium:
mass of Na = moles of Na x molar mass of Na
= 83.0 mol x 22.99 g/mol
= 1900 g
Therefore, 5.0 x 10²⁵ atoms of sodium have a mass of 1900 g.
3. The molar mass of Ni is 58.69 g/mol. Therefore, we can calculate the number of moles of Ni as:
moles of Ni = mass of Ni divided by molar mass of Ni
= 3.95 g / 58.69 g/mol
= 0.0673 mol
Then, we can use Avogadro's number to calculate the number of atoms of Ni:
number of atoms of Ni = moles of Ni x Avogadro's number
= 0.0673 mol x 6.022 x 10²³ atoms/mol
= 4.05 x 10²² atoms
Therefore, 3.95 g of Ni contain 4.05 x 10²² atoms.
4. The molar mass of Xe is 131.29 g/mol. Therefore, we can calculate the number of moles of Xe as:
moles of Xe = mass of Xe divided by molar mass of Xe
= 15 kg / 131.29 g/mol
= 114 mol
Then, we can use Avogadro's number to calculate the number of atoms of Xe:
number of atoms of Xe = moles of Xe x Avogadro's number
= 114 mol x 6.022 x 10^23 atoms/mol
= 6.87 x10²⁵ atoms
Therefore, a 15 kg sample of Xe gas contains 6.87 x 10²⁵ atoms.
5. A gas's molar volume at STP is 22.4 L/mol. Therefore, we can calculate the number of moles of Ar as:
moles of Ar = number of atoms of Ar divided by Avogadro's number
= 45,000,000 atoms / 6.022 x 10²³ atoms/mol
= 7.47 x 10⁻¹⁷ mol
Then, we can use the molar volume of a gas at STP to calculate the volume of Ar gas:
volume of Ar = moles of Ar x molar volume of gas at STP
= 7.47 x 10⁻¹⁷ mol x 22.4 L/mol
= 1.67 x 10⁻¹⁵ L
Therefore, 45,000,000 atoms of Ar would occupy a volume of 1.67 x 10⁻¹⁵ liters at STP.
6. The molar mass of CO₂ is 44.01 g/mol. Therefore, we can calculate the number of moles of CO₂ as:
moles of CO₂ = mass of CO₂ divided by molar mass of CO₂
= 75 g / 44.01 g/mol
= 1.70 mol
Then, we can use Avogadro's number to calculate the number of atoms of CO₂:
number of atoms of CO₂ = moles of CO₂ x Avogadro's number
= 1.70 mol x 6.022 x 10²³ atoms/mol
= 1.02 x 10²⁴ atoms
Therefore, a 75 g sample of CO₂ contains 1.02 x 10²⁴ atoms.
To know more about STP, visit:
https://brainly.com/question/29356493
#SPJ1
A company employs several high school students in its food-packaging factory. All the students are below 17 years of age. Which act prohibits
the employment of such persons?
OA. Occupational Safety and Health Act
OB. Fair Labor Standards Act
OC. Perishable Agricultural Commodities Act
OD. Homeland Security Presidential Directives
Answers
Answer:
Fair Labor Standards Act
Explanation:
It is correct. I just took the test on Clever
Answer:
I would say it’s B
Explanation:
What are the units for measuring specific heat?
Answers
Answer:
International system. The SI unit for specific heat is joule per kelvin per kilogram (J/K/kg, J/(kg K), J K−1 kg−1, etc.). Since an increment of temperature of one degree Celsius is the same as an increment of one kelvin, that is the same as joule per degree Celsius per kilogram (J/°C/kg).
can someone help me solve the questions below using the data table below please
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The percent by mass of acetic acid in the vinegar is 2.33%.
Below are the steps to solve the given problem using the data table given below:
Step 1: Calculate the mass of the vinegar. Given,Mass of flask and vinegar solution- 25.17gMass of flask- 15.12gMass of vinegar solution = Mass of flask and vinegar solution - Mass of flask= 25.17 g - 15.12 g= 10.05 g
Step 2: Calculate the moles of NaOH used in the titration.Molarity of NaOH solution is 0.1 M.Moles of NaOH = Molarity × Volume of NaOH usedMoles of NaOH = 0.1 M × 39.00 mL (since the initial volume of NaOH is 0.00 mL)Moles of NaOH = 0.0039 moles
Step 3: Determine the moles of acetic acid used in the reaction.The balanced chemical equation for the reaction between NaOH and acetic acid (the main component of vinegar) is given below:CH3COOH + NaOH → CH3COONa + H2OMoles of NaOH = Moles of CH3COOH (since they react in a 1:1 ratio)Moles of CH3COOH = 0.0039 moles
Step 4: Calculate the mass of acetic acid used in the reaction.Molar mass of acetic acid is 60.05 g/mol.Mass of CH3COOH = Moles of CH3COOH × Molar mass of CH3COOH= 0.0039 moles × 60.05 g/mol= 0.234 gStep 5: Calculate the percent by mass of acetic acid in the vinegar.Percent by mass of acetic acid = (Mass of CH3COOH / Mass of vinegar solution) × 100%= (0.234 g / 10.05 g) × 100%= 2.33%.
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
What is the uncertainty (in % v/v) of a solution prepared by pipetting 350 uL of ethanol using an Eppendorf pipet and diluting to the mark in a 10mL class A volumetric flask
Answers
The uncertainty (in % v/v) of the prepared solution is 0.00175%.
The uncertainty (in % v/v) of a solution prepared by pipetting 350 uL of ethanol using an Eppendorf pipet and diluting to the mark in a 10mL class A volumetric flask can be calculated as follows:
Uncertainty can be calculated by using the formula;
Uncertainty = (0.05/100) * V
Where, V is the volume measured in mL
The volume of ethanol measured is 350 μL = 0.35 mL
Therefore, the uncertainty = (0.05/100) * 0.35
= 0.000175 mL
The volume of the final solution is 10 mL
Therefore, the concentration of ethanol in the final solution is:
(0.35/1000) / (10/1000) = 0.035 g/mL or 3.5% v/v
The uncertainty can be expressed as a percentage of the concentration:
% uncertainty = (uncertainty / concentration) x 100
= (0.000175 mL / 10 mL) x 100
= 0.00175 % v/v
Therefore, the uncertainty (in % v/v) of the prepared solution is 0.00175%.
For such more questions on uncertainty
https://brainly.com/question/16941142
#SPJ11
The air mass over the northwest part of Texas is an area of ____________________; therefore, the weather associated with it is ______________________. Which words complete the sentence above?
A.) Low pressure ; stormy
B.) Low pressure ; clear
C.) High pressure ; stormy
D.) High pressure ; clear
Answers
Answer:
Low pressure ; stormy
Explanation:
calculate the mass of one atom of helium.
Answers
Answer:
One atom lf helium has a mass of 4 u
Know that 1 u =1.66. 10-24g
3. Briefly discuss the results of the TLC. Was there evidence of unreacted p-nitrobenzaldehyde in either product
Answers
TLC means Thin Layer Chromatography. It is a method that can best be described as "Affinity-Based" used in the separation of compounds that are in a mixture.
What is unreacted p-nitrobenzaldehyde?Unreacted p-nitrobenzaldehyde is simply an organic aromatic compound that contains a nitro group para-substituted to an aldehyde. in this case, if it is unreacted, that means it is the same as before the chemical reation.
Note that the question is missing key information hence the general answer.
Learn more about TCL at:
https://brainly.com/question/10296715
Match up the characteristics below with the type of molecular bond they describe. Bonds found in Halite (between Na+ and Cl-) Bonds found between Si and O in the Si-O tetrahedron Bonds inside the water molecule (between the H and O ) Bonds that exist between two water molecules Strongest bond type Weakest bond type Bonds that are used by water to dissolve sal
Answers
The characteristics and the type of molecular bond they describe:
1. Bonds found in Halite (between Na⁺ and Cl⁻): Ionic bond
2. Bonds found between Si and O in the Si-O tetrahedron: Covalent bond
3. Bonds inside the water molecule (between the H and O): Covalent bond
4. Bonds that exist between two water molecules: Hydrogen bond
5. Strongest bond type: Covalent bond
6. Weakest bond type: Van der Waals bond
7. Bonds that are used by water to dissolve salt: Ionic bond
The ionic bond is a type of molecular bond found in halite (between Na⁺ and Cl⁻). The Si-O tetrahedron is held together by a covalent bond. The bond inside the water molecule (between the H and O) is also a covalent bond. The hydrogen bond is the type of bond that exists between two water molecules. The covalent bond is the strongest bond type, while the van der Waals bond is the weakest bond type. Water uses the ionic bond to dissolve the salt.
Learn more about bonds: https://brainly.com/question/32306693
#SPJ11
How do you find the unknown compound in organic chemistry?
Answers
The unknown compounds are among a restricted set of substances that are provided for you in ascending mp and bp order. Applying your experimental results to these lists will help you narrow down the list of potential chemicals.
Compounds can be recognized by two tests, including
1. Physical assessment
2. Chemical assessment
Physical assessment
This is dependent on outward manifestations and qualities including State, Color, Texture, Smell (odor), Taste, and Feel.
Physical characteristics such as solubility, crystalline or amorphous nature Refractive index, melting point, and boiling point
pH, conductivity, functional groups, and other chemical assessment criteria. utilizing analytical approaches such as spectroscopy analysis and chromatography analysis.
To know more about unknown compound visit
https://brainly.com/question/28456436
#SPJ4
calculate the quantity of heat released in kj when 15.7 g of benzene in the liquid phase at 50.0 °c is converted to solid benzene at 2.0 °c. molar mass of benzene
Answers
The quantity of heat released in kJ when 15.7 g of benzene in the liquid phase at 50.0 °C is converted to solid benzene at 2.0 °C is 3.32 kJ.
What is the quantity of heat released?The quantity of heat released is calculated using the formula below:
The quantity of heat released = (heat evolved from 50 °C to 5.4 °C) + (latent heat of fusion) + (heat evolved on cooling from 5.4 °C to 2.0 °C)The following values apply to benzene:
Melting point = 5.4 °CBoiling point = 90.1 °CHeat of fusion = 9.9 kJ/molHeat of vaporization = 30.7 kJ/molSpecific heat (solid) = 1.51 J/g-"CSpecific heat (liquid) = 1.80 J/g "CSpecific heat (gas) = 1.92 J/g-°CMolar mass = 78.11 g/molMoles of benzene = 15.7/78/11 = 0.20 moles
Heat evolved from 50 °C to 5.4 °C = 15.7 * 1.8 * (50 - 5.4) = 1260.4 J = 1.26 kJ
Latent heat of fusion = 0.2 * 9.9 = 1.98 kJ
Heat evolved on cooling from 5.4 °C to 2.0 °C = 15.7 * 1.51 * (5.4 - 2.0) = 80.6 J = 0.08 kJ
quantity of heat released = 1.26 + 1.98 + 0.08 = 3.32 kJ
Learn more about quantity of heat at: https://brainly.com/question/19666326
#SPJ4
A metal object is to be gold-plated by an electrolytic procedure using aqueous AuCl3 electrolyte. How much gold may be deposited in 3.0 min by a constant current of 10. A
Answers
Answer:
This is the answer
Explanation:
charges passed = current x time = 10 x 3 x 60
= 1800 C
mole of charge = 1800 / 96500
= .01865 moles
Au+3 contains 3 positive charges
3 mole of charge will deposit 1 mole of Au .01865 moles will deposit .01865 / 3 mole
= 6.2167 x 10-3 moles.
which model of an atom is correctly labeled?

Answers
Answer:
B
DUH
The nucleus is made up of the protons (which have a positive charge) and neutrons and surrounded by a cloud of electrons ( which have a negative charge ).
Name the following hydrocarbons:

Answers
IUPAC nomenclature is a set of rules and guidelines established by the International Union of Pure and Applied Chemistry (IUPAC) for naming chemical compounds. The names of the given compounds are:
2-methyl, 2-hexene4-ethyl, 3,5-dimethyl, nonane4-methyl, 2-heptyne5-propyl decaneIUPAC naming provides a systematic and consistent approach to assigning unique and unambiguous names to chemical substances. It allows for effective communication and understanding among chemists worldwide. The IUPAC nomenclature covers a wide range of organic and inorganic compounds.
Learn more about IUPAC nomenclature, here:
https://brainly.com/question/14379357
#SPJ1
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
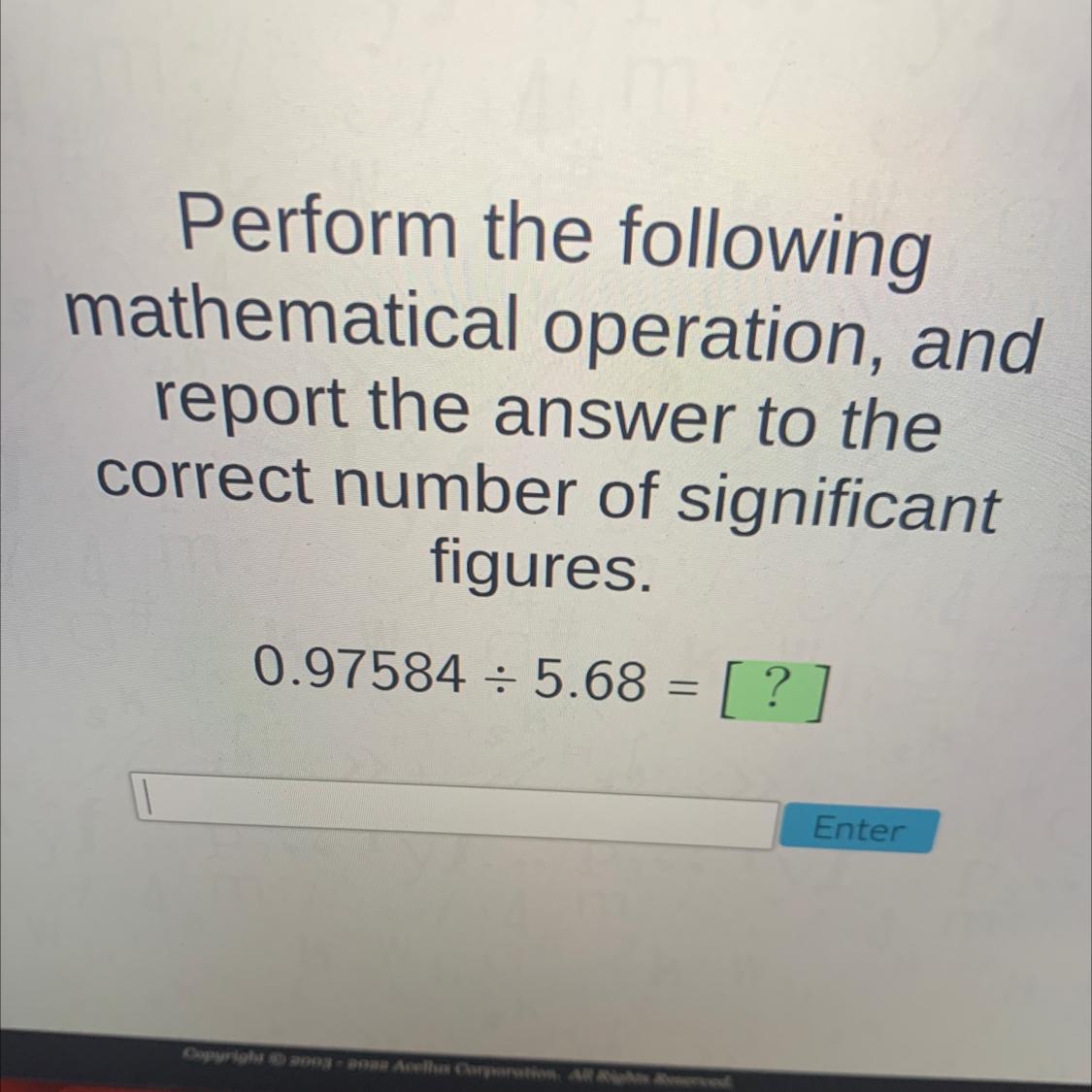
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Which receives the most solar radiation?
a. the oceans
b. the polar zones
C. the temperate zones
d. the tropics
Answers
Answer:
D
Explanation:
tropical areas receives the most solar radiation because the sun's rays are nearly perpendicular to the earths surface
Lorelai discovers a compound that is 64.8 g C, 13.62 g H, and 21.58 g O. What is the empirical formula of Lorelai's compound
Answers
The empirical formula of Lorelai's compound is C₄H₁₀O
Data obtained from the questionC = 64.8 gH = 13.62 gO = 21.58 gEmpirical formula =? How to determine the empirical formulaDivide by their molar mass
C = 64.8 / 12 = 5.4
H = 13.62 / 1 = 13.62
O = 21.58 / 16 = 1.35
Divide by the smallest
C = 5.4 / 1.35 = 4
H = 13.62 / 1.35 = 10
O = 1.35 / 1.35 = 1
Thus, the empirical formula of the compound is C₄H₁₀O
Learn more about empirical formula:
https://brainly.com/question/24297883
#SPJ1
The energy released in cellular respiration helps an athlete perform. How do you think an athlete might be able to increase cellular respiration?
Answers
Answer:
by eating healthy food and having a balanced diet also by exercising
An athlete might be able to increase their cellular respiration through the consumption of a balanced diet along with a regular exercising pattern.
What is Cellular respiration?Cellular respiration may be defined as a process through which organisms break down the molecules of glucose within the cell in order to avail energy in form of ATP for metabolic and cellular processes.
As we all know that glucose molecules and oxygen are the reactants for cellular respiration. So, a better level of glucose can be availed by a healthy and balanced diet and a standard oxygen level can be availed through a regular exercising pattern.
Therefore, an athlete might be able to increase their cellular respiration through the consumption of a balanced diet along with a regular exercising pattern.
To learn more about Cellular respiration, refer to the link:
https://brainly.com/question/2809259
#SPJ2
A solution of wheat flour and water has a [H+] of 1 × 10−8 M
Which type of solution is this?
O acidic
O neutral
O basic
Answers
In general, a solution with a [H+] of less than 1 × 10^-7 M is considered basic or alkaline, while a solution with a [H+] of greater than 1 × 10^-7 M is considered acidic. A [H+] of 1 × 10^-8 M is lower than the neutral value of 1 × 10^-7 M, indicating that the solution is basic.
Answer:
basic
Explanation:
How many molecules make up 8.41 moles of Ca2(SO3)?
Answer: ??? molecules Ca2(SO3) (no rounding; make sure to include scientific notation when necessary)
Answers
Answer:
5.06282 × 10²⁴ moleculesExplanation:
The number of molecules of Ca2(SO3) can be found by using the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
N = 8.41 × 6.02 × 10²³
We have the final answer as
5.06282 × 10²⁴ moleculesHope this helps you
How to get rid of high cholesterol?!
Please tell me
Answers
you have to drink a lot of water and 67890
10. a and b both are non-prime attribute. a determines b. a) not a 1 nf b) it can be a 1 nf, but not a 2 nf c) it can be a 2 nf, but not a 3 nf d) it can be a 3 nf, but not a bcnf
Answers
Based on the given information that attribute A determines attribute B, the correct option is (d) It can be in 3NF but not in BCNF.
To determine the normal form of a relation, we need to assess its functional dependencies. In this case, since attribute A determines attribute B, we have a functional dependency A -> B.
Not 1NF: This option is incorrect because A determining B does not violate the requirements of being in the first normal form (1NF). 1NF ensures atomicity of attributes and eliminates repeating groups.
It can be 1NF, but not 2NF: This option is incorrect because if A determines B, it satisfies the requirements of 2NF. 2NF mandates that non-prime attributes depend fully on the candidate keys, which is the case here. It can be 2NF, but not 3NF: This option is incorrect because if A determines B, it satisfies the requirements of 3NF. 3NF ensures that there are no transitive dependencies where non-prime attributes depend on other non-prime attributes.
It can be 3NF, but not BCNF: This option is correct. Although A determining B satisfies the conditions for being in 3NF, it may not meet the requirements for Boyce-Codd Normal Form (BCNF). BCNF requires that every determinant of a functional dependency is a candidate key, which may not be the case here.
Learn more atomicity here:
https://brainly.com/question/1566330
#SPJ11
list of electron geometry
Answers
Linear, trigonal planar, quadrilateral, tetragonal bipyramidal, or octahedral geometries are some examples of electron geometry. Although the molecule's form is changed, the configuration electrons unchanged.
Who or what is an electron?A negative charges new particle known as an electron can either be free or attached to an atom. One of the three principal kinds of particles inside an atom, along with neutrons and protons. is an elementary particle that is linked to the atom.
Why do electrons pass?Electrons are crucial to many physical events. The charge moves from the positive pole to the negative electrode of a battery owing to the electrical pressure caused by the voltage differential between the two terminals.
To know more about electrons visit:
https://brainly.com/question/15216746
#SPJ4
Compare a mixture and a compound. How are they alike?
Contrast a mixture and a compound. How are they different?
Answers
Answer:
gnzl8303
gnzl8303vvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvv
Explanation:
Answer:
how they are alike: Both compound and mixture are combined in a definite ratio or in any proportion. Both compound and mixture consist of two or more substances/elements. Both compounds and mixtures have physical and chemical properties.
how they are different: The chemical composition of compounds is always fixed. A mixture can have a variable composition of the substances forming it. Mixtures can either be homogeneous or heterogeneous in nature. The constituents of a compound can only be separated by either chemical or electrochemical methods (like extraction).
Explanation:
a solid sample of copper is an excellent conductor of electric current. which type of chemical bonds are in the sample ?
a.ionic bonds
b.metalic bonds
c.nonpolar bonds
d.polar bonds
Answers
Awnser
B. Metallic bonds
Explanation:
Castle Learning
Answer:
B) metallic bonds
Explanation:
Castle learning
