Answers
Answer:
okay I will help you just message me johnpatrick on messenger
Related Questions
Calculate the hydroxide ion concentration, [OH−]
, for a solution with a pH of 8.81
Answers
The hydroxide ion concentration of the given solution is 7.94 x 10⁻⁶ M.
The hydroxide ion concentration, [OH⁻], can be calculated from the pH of a solution using the equation:
pH + pOH = 14.
Since we have the pH of the solution as 8.81, we can first calculate the pOH as follows:
pOH = 14 - pH
pOH = 14 - 8.81
pOH = 5.19
Now, we can use the definition of pOH to calculate the hydroxide ion concentration:
pOH = -log[OH⁻]
5.19 = -log[OH⁻]
[OH⁻] = 7.94 x 10⁻⁶ M
The pH of a solution is a measure of its acidity or basicity, which is determined by the concentration of hydrogen ions, [H⁺], in the solution. A pH value of 8.81 indicates that the solution is slightly basic, meaning that it has a lower concentration of hydrogen ions and a higher concentration of hydroxide ions.
To learn more about hydroxide follow the link:
https://brainly.com/question/4251554
#SPJ1
What is the number of molecules of NO, which contains 16 gm of oxygen. 14
Answers
To find the number of molecules of NO that contains 16 g of oxygen, we need to first calculate the number of moles of oxygen in 16 g of oxygen. Using the atomic weight of oxygen (16 g/mol), we can calculate:
moles of O = mass of O / atomic weight of O = 16 g / 16 g/mol = 1 mol
Next, we need to determine the number of moles of NO that contains 1 mol of oxygen. From the molecular formula of NO, we can see that 1 mol of NO contains 1 mol of oxygen. Therefore, the number of moles of NO that contains 1 mol of oxygen is also 1 mol.
Finally, we can use Avogadro's number to convert the number of moles of NO to the number of molecules of NO. Avogadro's number is approximately 6.02 x 10^23 molecules/mol. Therefore, the number of molecules of NO that contains 16 g of oxygen is:
number of molecules of NO = number of moles of NO x Avogadro's number
number of molecules of NO = 1 mol x 6.02 x 10^23 molecules/mol
number of molecules of NO = 6.02 x 10^23 molecules
Therefore, there are approximately 6.02 x 10^23 molecules of NO that contain 16 g of oxygen.
What is the empirical formula of a compound that is 40.0% sulfur and 60.0% oxygen by weight?
Assume 100 g sample. g-->mol for each element, divide by smallest # of moles
S = 32 g/mol
O = 16 g/mol
Answers
Empirical formula is SO3 with one sulfur atom and three oxygen atoms.
What is empirical formula?Empirical formula is the chemical formula of compound that gives proportions (ratios) of the elements present in compound but not the actual numbers or arrangement of atoms.
For sulfur: 40.0% of 100 g = 40 g = 40 / 32 mol = 1.25 mol
For oxygen: 60.0% of 100 g = 60 g = 60 / 16 mol = 3.75 mol
The ratio of the number of moles of sulfur to the number of moles of oxygen is 1.25 mol / 3.75 mol = 1/3.
The empirical formula is therefore SO3 with one sulfur atom and three oxygen atoms.
To know more about empirical formula, refer
https://brainly.com/question/1603500
#SPJ1
At room temperature, potassium is classified as (1) a metallic solid (3) a network solid (2) a molecular solid (4) an ionic solid
Answers
at room temperature, potassium is a soft solid, classified as a metallic solid
At room temperature, potassium (K) is classified as a metallic solid. Therefore, option (1) is correct.
What is potassium?Potassium can be described as a chemical element with the chemical symbol K and atomic number 19. Potassium (K) is a silvery-white metal, it is soft and can be cut with a knife with little force.
Potassium reacts with atmospheric oxygen to form white potassium peroxide in only seconds of exposure. In the periodic table, potassium element is one of the alkali metals that have a single valence electron in the outer electron shell.
Potassium occurs only in the form of ionic salts in nature and reacts vigorously with water, generating sufficient heat. Potassium is a soft solid with a low melting point. It has metallic bonds in its lattice. The metallic bonds are formed between the electrons and positively charged potassium metal. Therefore, potassium is classified as a metallic solid.
Learn more about Potassium, here:
https://brainly.com/question/13321031
#SPJ2
What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
Radioactive decay of Mg, by graphical method prove that this is a first-order reaction
Answers
The first-order reaction is a chemical reaction in which the rate of the reaction depends on the concentration of only one reactant. The radioactive decay of Mg is an example of a first-order reaction.
To prove that the radioactive decay of Mg is a first-order reaction, we can graph the natural logarithm of the remaining concentration of Mg versus time. The resulting graph should be a straight line with a negative slope, according to the mathematical expression for a first-order reaction.
If we measure the concentration of Mg at different times, we can calculate the remaining concentration of Mg and take its natural logarithm. Then, we can plot these values against time. If the resulting graph is a straight line with a negative slope, this indicates that the reaction is first-order.
Experimentally, we can measure the radioactivity of Mg at different times and calculate the concentration of Mg. Then, we can plot the natural logarithm of the remaining concentration of Mg versus time. If the resulting graph is a straight line with a negative slope, this confirms that the radioactive decay of Mg is a first-order reaction.
To know more about the Radioactive, here
https://brainly.com/question/31088907
#SPJ1
Which subatomic particle has the least mass?
electron
proton
neutron
the atom
Answers
Answer:
election
Explanation:
write a skeletal equation for the following reaction: An aqueous solution of barium nitrate and an aqueous solution of sodium sulfate are mixed together. Then balance the completed chemical equation.
Answers
Answer:
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + 2 NaNO₃(aq)
Explanation:
Let's consider the unbalanced double displacement equation for the reaction of an aqueous solution of barium nitrate and an aqueous solution of sodium sulfate.
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + NaNO₃(aq)
To get the balanced equation, we will multiply NaNO₃(aq) by 2.
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + 2 NaNO₃(aq)
A sample of trifluoroacetic acid, C2HF3O2, contains 77.7 g of fluorine. Calculate the mass of the trifluoroacetic acid sample.
Answers
The mass of trifluoroacetic acid, C₂HF₃O₂ which contains 77.7 g of fluorine is 155.4 grams
How do I determine the mass of trifluoroacetic acid, C₂HF₃O₂?We'll begin by obtaining the mass of C₂HF₃O₂ in one mole. Details below:
1 mole of C₂HF₃O₂ = (12 × 2) + 1 + (19 × 3) + (16 × 2) = 114 gMass of fluorine, F in 1 mole of C₂HF₃O₂ = 3F = 3 × 19 = 57 gThus, we can say:
57 grams of fluorine, F are present in 114 grams of C₂HF₃O₂
With the above information, we can obtain the mass of C₂HF₃O₂ that contains 77.7 g of fluorine. Details below:
57 grams of fluorine, F are present in 114 grams of C₂HF₃O₂
Therefore,
77.7 grams of fluorine, F will be present in = (77.7 grams × 114 grams) / 57 grams = 155.4 grams of C₂HF₃O₂
Thus, from the above calculation, we can conclude that the mass of trifluoroacetic acid, C₂HF₃O₂ is 155.4 grams
Learn more about mass composition:
brainly.com/question/9274824
#SPJ1
Can anyone help me? :)
Worth 30 points to first right answer!

Answers
A 52gram sample of water that has an initial temperature of 10C absorbs 4130 joules. If the specific heat of water is 4.184 j what is the final temperature of the water?
Answers
A 52gram sample of water that has an initial temperature of 10C absorbs 4130 joules. If the specific heat of water is 4.184 j. 25°C is the final temperature of the water.
The physical concept of temperature indicates in numerical form how hot or cold something is. A thermometer is used to determine temperature. Thermometers are calibrated using several temperature scales, which traditionally drew on different reference locations.
The most popular scales include the Celsius scale, sometimes known as centigrade, with the unit symbol °C, the scale of Fahrenheit (°F), or the Kelvin scale (K), with the latter being mostly used for scientific purposes.
q = m×c×ΔT
4130 = 52×4.184×(T2- 10C)
T2= 25°C
To Know more about temperature, here:
https://brainly.com/question/11464844
#SPJ1
Can anybody answer this question of chemistry?

Answers
Answer:
Answer:A
Answer:AExplanation:
Answer:AExplanation:Molar Mass of glucose = (6×12)+(1×12)+(16×6)= 180g/mol
= 180g/molNumber of moles of Glucose = Mass/Molar Mass
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556= 46.5×55.5556
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556= 46.5×55.5556= 2555.55
Answer:
Answer:A
Answer:AExplanation:
Answer:AExplanation:Molar Mass of glucose = (6×12)+(1×12)+(16×6)= 180g/mol
= 180g/molNumber of moles of Glucose = Mass/Molar Mass
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556= 46.5×55.5556
= 180g/molNumber of moles of Glucose = Mass/Molar Mass= 5000/180= 27.7778molesIn the balanced equation of fermentation, the ratio of glucose to ethanol is 2:1Therefore the number of moles of ethanol is 2×27.7778=55.5556molesMass of ethanol= Molar Mass of ethanol × Number of moles={(12×2)+(1×6)+16} × 55.5556= 46.5×55.5556= 2555.55
This is a diagram of fossils found in the same area. Jamie wanted to know which layer of fossils is the oldest, so he
asked his mom. Which layer of fossils did Jamie's mom tell him was the oldest?
A)
Layer 1
B)
Layer 2
C)
Layer 3
D)
Layer 4

Answers
Answer:
D
Explanation:
The bottommost layer is always the oldest.
Laboratory balances that measure to the hundredths (0.01g) are calleda) centigram balanceb) milligram balancec) analytical balance
Answers
Answer:
\(A\)Explanation:
Here, we want to select the balance used to measure mass to a hundredth accuracy
The kind of balance used in this case is the centigram balance. The measure the mass of a substance to an accuracy of a hundredth which is 0.01g
Select the best answer for the question.
11. Iron(II) is available to bond with chloride ion. How many of each type of ion will bond to form an ionic compound?
O A. 2 iron(II), 3 chloride
OB. 1 iron(II), 2 chloride
C. 2 iron(II), 1 chloride
D. 3 iron(II), 1 chloride
Answers
The correct answer is A. 2 iron(II), 3 chloride, that is 2 iron(II) ions and 2 chloride ions will bond to form an ionic compound
In an ionic compound, the total positive charge of the cations should balance the total negative charge of the anions. Iron(II) is a cation with a charge of +2 (Fe2+), while chloride is an anion with a charge of -1 (Cl-).
We need to balance the charges and hence need to determine the least common multiple (LCM) of the charges. The LCM of +2 and -1 is 2. Therefore, we need two chloride ions (2 x -1 = -2) to balance the charge of one iron(II) ion (+2).
Hence, for each ionic compound formed, we would need 2 iron(II) ions and 2 chloride ions to achieve charge neutrality. Therefore, the correct answer is A. 2 iron(II), 3 chloride.
for more questions on compound
https://brainly.com/question/29108029
#SPJ11
Problem PageQuestion Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O. Suppose 3.3 g of hydrochloric acid is mixed with 5.00 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Round your answer to 2 significant digits.
Answers
Answer:
Mass of Nacl = 4.92 gram (Approx)
Explanation:
Given:
Hydrochloric acid = 3.3 g
Sodium hydroxide = 5 g
Computation:
Hcl + NaoH ⇒ Nacl + H₂O
Number of mole of Hcl = 3.3 / 36.46 = 0.0905 moles
Number of mole of NaoH = 5 / 40 = 0.125 moles
We, know that number of moles in Hcl is less then number of mole in NaoH
So,
Number of mole of Hcl = Number of mole of Nacl
So,
Mass of Nacl = Number of mole of Nacl × Molar mass of Nacl
Mass of Nacl = 0.0905 × 54.4
Mass of Nacl = 4.92 gram (Approx)
C11H24 how would you call is it decane or?
Answers
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).The gravitational pull of the Moon is much less than the gravitational pull of Earth. Consider an object with a ma of 10 kilograms that weighs 22 pounds on Earth Which statement describes the object on the Moon? A. Its mass would be much greater. B. lts weight would be much greater OOOO C. Its we ght would be the same O D. Its mass would be the same.
Answers
Answer: D.
Explanation: I got it right and the dude above me is spreading fake news
can two objects have the same mass and volume but be made of a different substance?explain.
Answers
Answer:
Yes
Explanation:
The two different material would have the same density but different chemical properties. Melting point/ boiling point etc would likely be different between the two materials too.
HELP PLEASE HERE IS THE QUESTION The shape of an apple is a CHEMICAL OR A PHYSICAL CHANGE OR CHEMICAL OR A PHYSICAL PROPITRY.
Answers
Answer:
Physical Property
Explanation:
As there is no chemical change or chemical related stuff in it!
Answer:
physical
Explanation:
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
is Al+CuCl2 a spontaneous reaction?
Answers
Answer:
No, it isn't a spontaneous reaction.
Explanation:
A spontaneous reaction is a reaction that requires no outside intervention to start.
All chemical reactions require energy.
mark brainliest please!
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
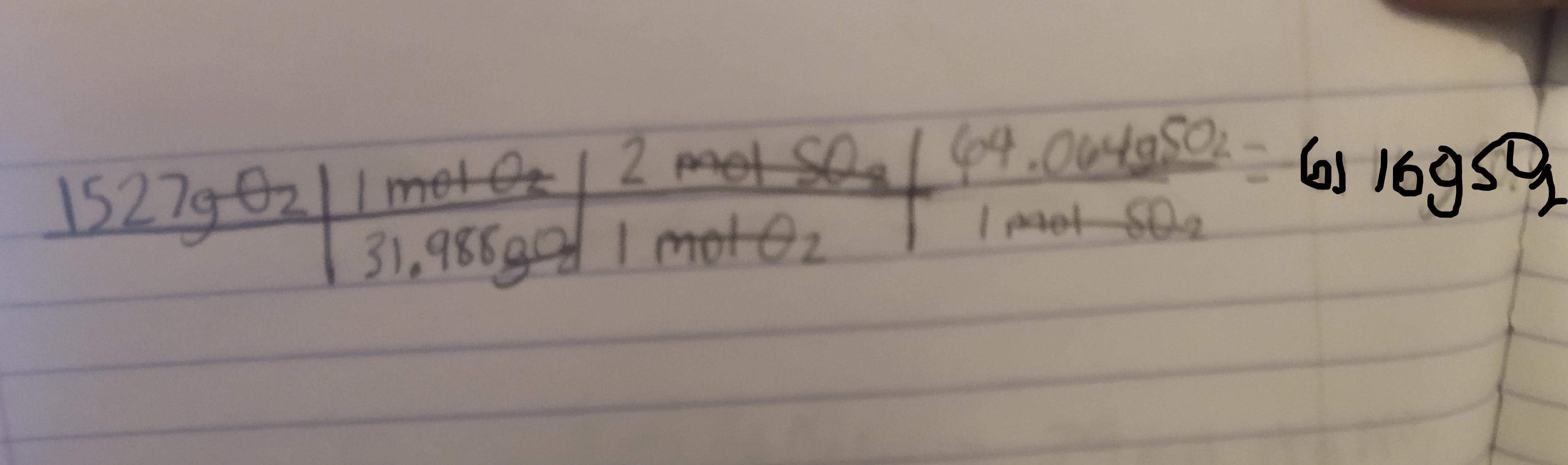
Silver chloride was reacted with magnesium hydroxide, in which magnesium displaced silver
ced the following reaction and state which one will form a precipitate.. (6mks)
Answers
Answer:
The precipitate is MgCl2Explanation:
The reaction that is described goes as follows:
2AgCl + Mg(OH)2 ---> MgCl2 + 2AgOH
The precipitate here is the MgCl2 salt.
I hope it helped!
Estimate the salt-to-water mass ratio that would produce the necessary change in temperature (i.e., 22-25°C.) Use the mass of salt calculated in the previous question and the water-to-salt ratio to determine the volume of water needed.
Answers
Answer:
Sea water salinity is expressed as a ratio of salt (in grams) to liter of water. In sea water there is typically close to 35 grams of dissolved salts in each liter. It is written as 35‰. The normal range of ocean salinity ranges between 33-37 grams per liter (33‰ - 37‰).
Suppose you were doing a titration where you start out with a basic solution of around 8.0 and you expect to keep adding an acid until the mixture has a pH of 3.0. Based on the indicator chart which pH indicator would be the best one to use. Describe the color change that would be observed

Answers
Based on the indicator chart, the pH indicator that would be the best one to use would be Thymol Blue.
The color change would be from light blue to yellow to orange.
Why is this pH indicator best ?Thymol blue would be best because it would show you where your starting point is and then when you reach the desired pH value of 3. 0. Looking at the indicator chart, Thymol blue has a color of light blue between 8. 0 and 9. 0 so you will know you are at 8. 0 when the reaction starts.
As you add more acid, the color would move to yellow to let you know that it is getting more acidic. Once it reaches orange, the titration should stop.
Find out more on pH indicators at https://brainly.com/question/29584301
#SPJ1
5. If 5.00cm³ of 0.20 mol.dm Na2CO3 was diluted with distilled water to obtain 250cm³ solution. What is the concentration of the resulting solution?
Answers
If 5.00cm³ of 0.20mol/L Na\(_2\)CO\(_3\) was diluted with distilled water to obtain 250cm³ solution. 0.64 mol/L is the concentration of the resulting solution.
What is concentration?Concentration in chemistry refers to the quantity of a material in a certain area. The ratio of the solute in the solution to the solvent or whole solution is another way to define concentration.
In order to indicate concentration, mass every unit volume is typically used. The solute concentration can, however, alternatively be stated in moles or volumetric units. Concentration may be expressed as per unit mass rather than volume.
Concentration₁×volume₁=concentration₂×volume₂
0.20×5.00=concentration₂×250
concentration₂= 0.64 mol/L
Therefore, 0.64 mol/L is the concentration of the resulting solution.
To know more about concentration, here:
https://brainly.com/question/30462414
#SPJ9
50 points, and I’ll mark as brainliest!!!!!
Tasks are in the picture.

Answers
pH determines the acidic or alkaline a solution is using the pH scale, which has a range of 0 to 14. An alkaline pH is greater than 7, while an acidic pH is less than 7.
Thus, The pH of a solution is defined mathematically as the negative logarithm of the molar concentration of hydrogen ions therein.
NaOH is a strong alkaline, as indicated by a pH testing strip, but in order to determine its exact pH, you must first determine its molarity.
A scale known as pH is used to describe how basic or acidic a water-based solution is. Basic solutions have a higher pH than acidic solutions, which have a lower pH.
Thus, pH determines the acidic or alkaline a solution is using the pH scale, which has a range of 0 to 14. An alkaline pH is greater than 7, while an acidic pH is less than 7.
Learn more about pH, refer to the link:
https://brainly.com/question/15289741
#SPJ1
The pH of HNO₂ is 2.15, pH of NH₄OH is 10.98 and pH of H₂S is 3.76.
pH is defined as the negative logarithm of H⁺ ion concentration.
pH is a measure of how acidic or basic a substance is. In our everyday routine, we encounter and drink many liquids with different pH. Water is a neutral substance. Soda and coffee are often acidic.
The pH is an important property, since it affects how substances interact with one another and with our bodies. In our lakes and oceans, pH determines what creatures are able to survive in the water.
Given,
1. Concentration = 0.1
Ka = 4.5 × 10⁻⁴
\(pH = \frac{1}{2} (pka - log c)\)
pH = 0.5 × ( 3.3 + 1)
= 2.15
2. Concentration = 0.05
Ka = 1.8 × 10⁻⁵
\(pOH = \frac{1}{2} (pkb - log c)\)
pOH = 0.5 × ( 4.74 + 1.3)
= 3.02
pH = 14 - pOH
= 14 - 3.02
= 10.98
3. Concentration = 0.3
Ka = 1 × 10⁻⁷
\(pH = \frac{1}{2} (pka - log c)\)
pH = 0.5 × ( 7 + 0.52)
= 3.76
Learn more about pH, here:
https://brainly.com/question/15289714
#SPJ1