Answers
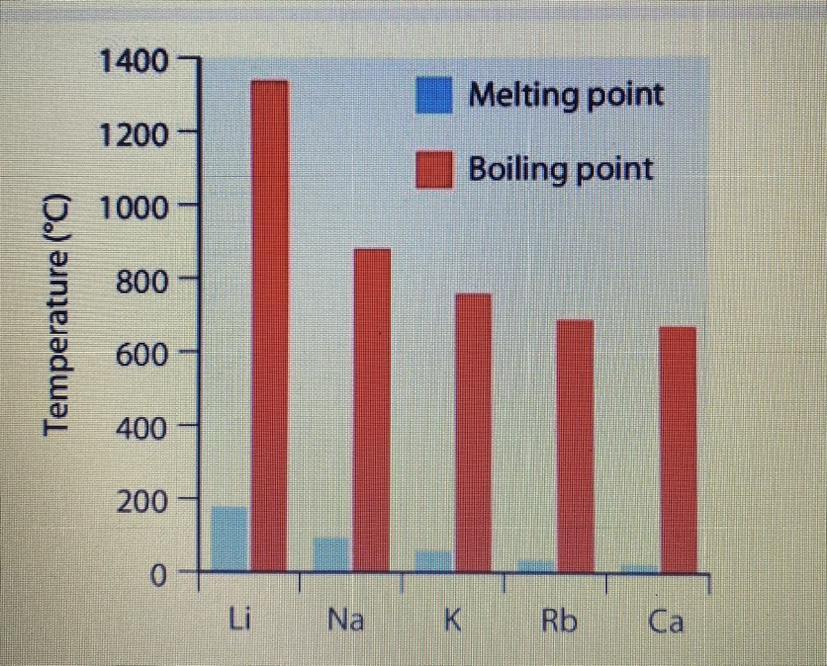
The melting point of francium was thought to be around 8.0 °C (46.4 °F); Additionally, 27 °C (81 °F) is frequently encountered. Due to its radioactivity and extreme rarity, the element's melting point is unknown. 20 1.5 °C (68.0 2.7 °F) was the result of a different extrapolation based on Dmitri Mendeleev's method.
Why is francium so soft to the touch?There are additional electron shells as you move down any group—for instance, Lithium has two shells and Francium has seven—that result in electron shielding. The force of attraction diminishes as it travels further through these shells to the outermost one, resulting in less powerful bonding.
Why do alkali metals have lower melting and boiling points?The weaker each metallic bond becomes, the lower the melting and boiling points become. The iotas in a metal are kept intact by the fascination of the cores to electrons which are delocalized over the entire metal mass.
To learn more about melting point here
https://brainly.com/question/29578567
#SPJ1
Related Questions
What strucrutal information can we gather from NMR spectroscopy? Can NMR alone determine the structure of a molecule?
Answers
Answer:
NMR reveals the chemical environment of atoms in a molecule
Explanation:
Nuclear magnetic resonance is a choice method among chemists when attempting to decipher the structure of a molecule.
There are several atoms whose nuclei are NMR active. Such nuclei include; H-1, C-13, etc.
The splitting pattern in an NMR spectrum gives important information about the chemical environment of atoms in the molecule.
However, NMR alone can not solely give all the required information about the structure of a molecule.
Please Answer Giving Brainleist
What gas in the air is used by plants to make their own food?
A) Nitrogen
B) Water vapor
C) Carbon dioxide
D) Oxygen
Answers
Answer:
C) Carbon dioxide is the answer.
Answer:
C. Carbon Dioxide
Carbon is absorbed by plants and chemically converted to oxygen via photosynthsis.
Hope this helps!
CH3 – CH2 – CH2 – CH2 – OH
Answers
Answer:
IUPAC name is butanol
I hope it's helps you
use the equation: 2Mg + O2→ 2MgO
Calculate the number of moles of oxygen needed to react with 6.00 moles of Mg.
Answers
Answer:
3 moles of O2 needed
Explanation:
2 moles of Mg to one mole O2
so 3 moles of O2 needed
Empty crucible and lid
Crucible, lid and Mg
Crucible, lid, and combustion product
42.39g
42.66g
0419 Mo
42.39-41.98
1. (2 pts) Determine the mass of the magnesium used.
Directions: Show all work in the space provided and write your answers on the lines with correct
significant figures and units! Using the data below please perform the following calculations:
Mass:
41.98g
0.00168 mil Ma
2. (2 pts) Determine the number of moles of magnesium used.
0.410Mg
24 3glnel
3. (2 pts) Determine the mass of magnesium oxide formed.
0.41g Mg
0.0256g Mg0
0.0072150
10 g
0.0184 -0.0256
4. (2 pts) Determine the mass of oxygen that combined with the magnesium.
5. (2 pts) Calculate the number of moles of oxygen atoms that were used.
= 1 My
07. For 60
Copyright © 2018 MsRazz ChemClass
6. (2 pts) Calculate the ratio between moles of magnesium used and moles of oxygen used.
ON mil Ma
0.0184 m My
0.0073
O(£។
7. (2 pts) Based on your experimental data, write the empirical formula for magnesium oxide.
1
8. (2 pts) Calculate the percent error in your determination of the magnesium: oxygen mole ratio,
using 1.00 as the accepted value.

Answers
We can compute a number of values that are connected to the experiment's magnesium and oxygen thanks to the provided data. By deducting the mass of the empty crucible and lid from the mass of the crucible, lid, and magnesium, we can first calculate the quantity of magnesium that was really utilised.
The weight that results is 0.27 grammes. We determine that this equates to around 0.0111 moles of magnesium using the molar mass of magnesium.The mass of the magnesium utilised is subtracted from the mass of the crucible, lid, and combustion product to get the mass of magnesium oxide that is created. Magnesium oxide's mass is determined to be 0.41 grammes.
We may infer from this data that the combined mass of magnesium and oxygen is We may infer from this data that the combined mass of oxygen and magnesium is 0.14 grammes. We calculate the amount of oxygen atoms utilised in the process as 0.00875 moles by dividing this mass by the molar mass of oxygen.
The empirical formula for magnesium oxide, MgO, is then determined to be roughly 1.27 moles of magnesium to moles of oxygen.The error percentage in calculating the magnesium-to-oxygen mole ratio is finally calculated, and it is discovered to be roughly 27%.
In conclusion, 0.27 grammes, or 0.0111 moles, of magnesium were consumed. 0.41 grammes of magnesium oxide were produced. The mass of the oxygen and magnesium together was 0.14 grammes, or 0.00875 moles.
Learn more about magnesium at :
https://brainly.com/question/8351050
#SPJ1
The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after an 8 h immersion period. What is the corresponding corrosion current density in mA/em2, assuming that all the corrosion is due to the reaction:
Al → Al3+ + 3e
The atomic weight of Al is 26.98 g/mol.
Answers
Answer:
I = 0.0931 A/cm^2 or 93.1 mA/cm^2
Explanation:
The computation of the corresponding corrosion is shown below:
As we know that
The mathematical form is
\(m=\frac{Q}{F} \frac{M}{z}\)
where,
m = substance mass
Q= total electric charge
F= Faradays constant i.e. = 96,500 C/mol
M = Substance molar mass
z = number of electrons transferred
Now
Q = It
where
I = current
And t = time
\(m=\frac{Q}{F} \frac{M}{z}\)
So,
\(I = \frac{mFz}{tM}\)
Now it is mentioned that
z=3, M=26.98 g/mol, m=0.25 g/cm2
So,
\(I= \frac{0.25 g/cm^2 \times 96,500 C/mol \times 3}{((8\times60\times60 s)} \times 26.98 g/mol)\)
Hence,
I = 0.0931 A/cm^2 or 93.1 mA/cm^2
Strontium-90 is present in radioactive fallout and has a half-life of 28.8 years. The first atomic detonation was at a place called Trinity Site in New Mexico in 1945 - 68 years ago. What percentage of Strontium-90 would remain in the soil at the site?
Round your answer to the nearest 5%. Example: If your answer would be greater than or equal to 32.5%, and less than 37.5%, you would report 35 - do not use a decimal or percent sign (%) after the number.
Answers
About 2.5% of Strontium-90 would remain in the soil at the Trinity Site.
Since the half-life of Strontium-90 is 28.8 years, we can use the formula for radioactive decay to calculate the percentage of Strontium-90 that would remain in the soil after 68 years:
Percentage remaining = (1/2)^(68/28.8) x 100
Solving this equation, we get:
Percentage remaining = 2.5%
This means that after 68 years, only 2.5% of the original amount of Strontium-90 would remain in the soil at the Trinity Site. The rest of the Strontium-90 would have decayed into other elements through radioactive decay. It's important to note that Strontium-90 is a dangerous radioactive isotope that can cause cancer and other health problems if ingested or inhaled, so measures should be taken to prevent people from being exposed to it.
To learn more about radioactive decay, here
https://brainly.com/question/1770619
#SPJ1
A golfer putted a golf ball 4.7 ft across a green. How many inches does this represent?
HOW DO WE GET THERE?
Since we are trying to convert 4.7 ft to inches, what is the appropriate conversion factor we must use in this case?
1 ft
12 in.
12 in.
1 ft
Answers
To find out how many inches are in 4.7 ft, you have to multiply 4x12 because there are 12 in in a ft. Since 4x12=48, just at the 7 in to 48 and you’ll get 55 in.
equipment needed to measure moles in chemistry?
Answers
The equipment needed to measure moles in chemistry would be the weighing balance.
Moles of substancesIn chemistry, the mole of a substance is the ratio of the mass of the substance and its molar mass. It is otherwise known as the number of moles in substances and can be mathematically expressed as:
Mole = mass/molar mass
Thus, in order to find the number of moles present in a substance, the mass of the substance must be known and the molar mass is calculated appropriately.
Since the molar mass of substances is determined from the sum of molar masses of atoms of elements present in the chemical formula of the substance, the molar mass is theoretical.
However, to find the mass of the substance, it has to be weighed using a suitable measuring scale, usually in the form of a weighing balance.
More on moles of substances can be found here: https://brainly.com/question/14200405
#SPJ1
Iron is generally produced from iron ore through the following reaction in a blast furnace. If400 g of Fe2O3 are available to react, how many moles of CO are needed?Fe2O3 + 3C0 + 2Fe + 3CO2
Answers
Step 1 - Understanding the relation of moles
The reaction between Fe2O3 and CO is:
\(Fe_2O_{3(s)}+3CO_{(g)}\rightarrow2Fe_{(s)}+3CO_{2(g)}\)We can see in this equation that 1 mole of Fe2O3 consumes 3 moles of CO. This is a fixed proportion that we'll be using to solve the problem.
Step 2 - Finding how many moles of Fe2O3 have reacted
To discover how many moles (n) of Fe2O3 have reacted, we can divide the mass (400g) by the molar mass of Fe2O3 (159 g/mol):
\(n=\frac{m}{M}=\frac{400}{159}=2.51\text{ moles}\)Step 3 - Finding how many moles of CO are needed
Now that we know how many moles of Fe2CO3 reacted, we can set the following proportion:
\(\begin{gathered} 1\text{ mole of Fe2O3 consumes --- 3 moles of CO} \\ 2.51\text{ moles of Fe2O3 consume ---- x} \\ \\ x=\frac{3\times2.51}{1}=7.53\text{ moles of CO} \end{gathered}\)Therefore, 7.53 moles of CO are needed.
Note: Finding the molar mass of Fe2O3
Multiply the molar mass of each element by the number of times it appears in the formula and then sum it all up. The molar masses are 16 g/mol for O and 56 g/mol for Fe. We have thus:
\(\begin{gathered} O\rightarrow3\times16=48 \\ \\ Fe\rightarrow2\times56=112 \end{gathered}\)The molar mass will be thus:
\(M(Fe2O3)=48+112=160\text{ g/mol}\)Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
A balanced chemical equation has equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of
Answers
Answer:
conservation of mass
hydrogen bond is not formed between, a)nitrogen and hydrogen b)carbon and hydrogen c) filorine and hydrogen d) iodine and hydrogen
Answers
Hydrogen bond is not formed between carbon and hydrogen. That is option B.
What is hydrogen bond?Hydrogen bond can be defined as the type of bond that is characterized to be a weak force that forms a special type of dipole-dipole attraction which occurs when a hydrogen atom bonded to a very electronegative atom such as a Nitrogen, Oxygen or Fluorine.
The combination of carbon and hydrogen is through a chemical bond called covalent bond which can occur between carbon and hydrogen atoms that can be found in many organic compounds (these are compounds that are made up of mainly carbon and hydrogen atoms).
Learn more about chemical bond here:
https://brainly.com/question/30688215
#SPJ1
the human population grew form 1 billion in the year 1800to blank billion in the year 200
Answers
The human population grew from 1 billion in the year 1800 to approximately 7.8 billion in the year 2021.
In the year 1800, the estimated global human population was around 1 billion. Over the next two centuries, significant advancements in technology, medicine, agriculture, and improved living conditions contributed to a rapid increase in population.
The growth rate of the human population began to accelerate in the 20th century. By the year 1927, the global population reached 2 billion. It took just 33 years for the population to double, reaching 4 billion in 1960. The population continued to grow at an unprecedented rate, with 6 billion people on Earth by the year 1999. As of 2021, the estimated global population stands at approximately 7.8 billion.
This remarkable growth in population can be attributed to several factors, including advancements in healthcare leading to reduced infant mortality rates, improved access to education and contraception, increased agricultural productivity, and overall socio-economic development.
It's important to note that population growth has not been uniform across all regions. Different countries and regions have experienced varying rates of population growth due to factors such as fertility rates, mortality rates, migration patterns, and government policies.
For more such questions on population visit:
https://brainly.com/question/30148263
#SPJ8
Calculate the volume of 0.5 M HCOOH and 0.5 M HCOONa required to prepare 0.1 L of pH 4.50 buffer with a buffer strength of 0.1 M. The pa of HCOOH is 3.75.
Answers
The volume of 0.5 M HCOOH required is 50 mL and the volume of 0.5 M HCOONa required is 150 mL.
To prepare a pH 4.50 buffer with a buffer strength of 0.1 M, we need to use a mixture of HCOOH and HCOONa in a specific ratio. The buffer equation for this system is:
\(pH = pKa + log([HCOO-]/[HCOOH])\)
Substituting the given pH of 4.50 and pKa of 3.75:
We can then use the Henderson-Hasselbalch equation:
\([HCOOH]/[HCOO-] = 3.16 \\\\[HCOOH] + [HCOO-] = 0.1 M\)
Solving these equations simultaneously, we get:
\([HCOOH] = 0.0253 M\) and \([HCOO-] = 0.0747 M.\)
Therefore, the volume required is 50 mL and the volume of 0.5 M HCOONa required is 150 mL, to prepare 0.1 L of pH 4.50 buffer with a buffer strength of 0.1 M.
To know more about Henderson-Hasselbalch equation, here
brainly.com/question/30466186
#SPJ1
Question 3 (0.2 points)
Since thermal energy represents the total energy of the particles in an object, at the
same temperature, the larger object will possess more thermal energy.
True
False
Answers
Why is the H-I bond more polar than the S-S bond?
Answers
Answer:
see explanation
Explanation:
The polarity of bonds is determined by electronegativity differences. As a guideline we define bonds as:
ionic if Δχ > 2.0
polar if 2.0 > Δχ > 0.5
nonpolar if 0.5 > Δχ
(X being electronegativity)
The polarity of bonds helps us understand non-covalent forces between molecules, such as hydrogen bonding and dipole-dipole interactions. It also helps us interpret the reactivity of molecules. For example, the Si-H bond (χSi = 1.8, χH = 2.1) is more hydride-like than the C-H bond (χC = 2.5, χH = 2.1). Therefore silanes react with acids to make H2, whereas phosphines (χP = 2.1) and hydrocarbons do not. Similarly, electrophilic substitution reactions occur more readily on Si-H and P-H compounds than they do on C-H compounds.
Does modern industrial agriculture have a sustainable future?
Answers
Advances in modern agriculture allow today's farmers to grow in ways that are measurably more sustainable. These practices help farmers retain topsoil and reduce erosion, conserve water in multiple ways, reduce emissions, protect pollinators, and protect natural resources by using farmland more efficiently. Industrialized agriculture is highly concentrated and mechanized, relying on chemical inputs like fertilizers, pesticides and non-therapeutic antibiotics. Agriculture often places significant pressure on natural resources and the environment.
13. A compound with a molar mass of 78.0 g/mol is found to contain 92.29%
carbon and 7.71% hydrogen, by mass. The molecular formula of the compound is
a. CH
b. C₂H3
c. C3H3
d. C5H₁
e. C6H6
Answers
The molecular formula of the compound is C6H6
Molecular formulaFirst, convert the percentages to moles
C = 92.9/12 = 7.74
H = 7.71/1 = 7.71
Thus, the empirical formula will be CH
[empirical formula]n = molecular formula
Empirical formula mass = 12 + 1 = 13
n = empirical formula mass/molar mass = 78/13 6
Thus, the molecular formula will be C6H6
More on molecular formulas can be found here: https://brainly.com/question/14425592
#SPJ1
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
draw the structure of Benzanal
Answers
Answer:
check your question it's not correct
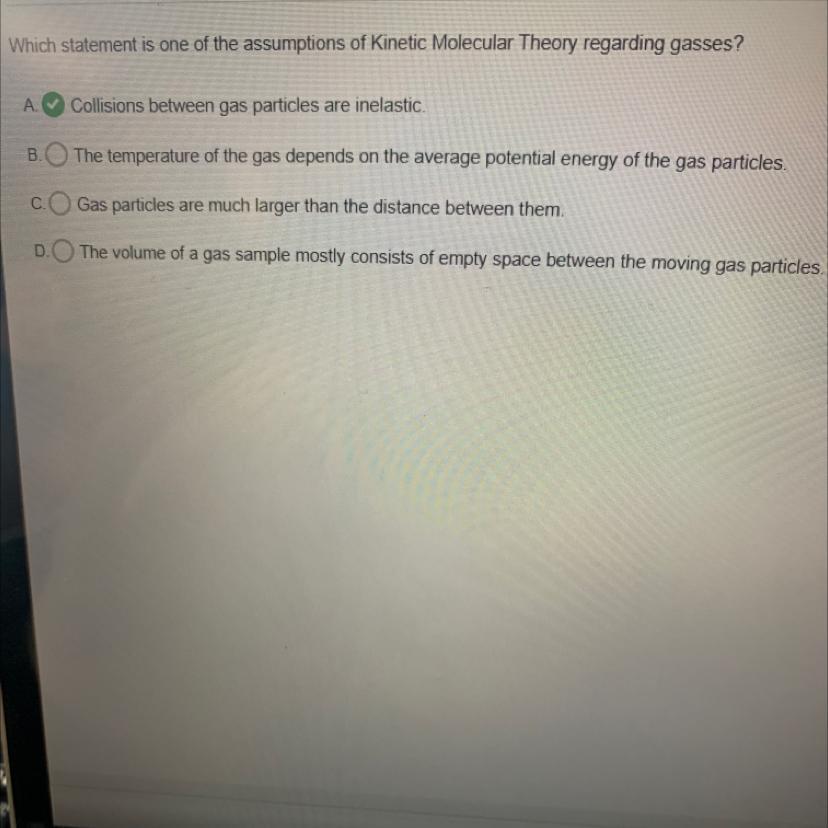
Which statement is one of the assumptions of Kinetic Molecular Theory regarding gasses?
A Collisions between gas particles are inelastic.
The temperature of the gas depends on the average potential energy of the gas particles.
Gas particles are much larger than the distance between them.
The volume of a gas sample mostly consists of empty space between the moving gas particles.
Answers
The statement that "The volume of a gas sample mostly consists of empty space between the moving gas particles" is one of the assumptions of the Kinetic Molecular Theory regarding gases.
What is Collision?
There are different types of collisions, depending on the nature of the objects involved, the speed and direction of their motion, and the type of contact that occurs. For example, elastic collisions are those in which the total kinetic energy of the colliding objects is conserved, meaning that no energy is lost or gained during the collision. In contrast, inelastic collisions are those in which some of the kinetic energy is transformed into other forms of energy, such as heat or sound.
The Kinetic Molecular Theory is a model that describes the behavior of gases. One of the main assumptions of this theory is that gas particles are in constant random motion and move in a straight line until they collide with other particles or the walls of their container.
Another important assumption of this theory is that the volume of a gas sample mostly consists of empty space between the moving gas particles. This means that gas particles are assumed to be very small compared to the overall volume of the gas sample. Therefore, the particles do not occupy all of the available space in the container, but instead only occupy a small portion of it.
Learn more about Collision from given link
https://brainly.com/question/24915434
#SPJ1
How many moles of O2 are in 6.7 moles of H2O
Answers
1) Find moles of oxygen (02)
2 molecules of water (H2O) contain 1
molecule of oxygen (02).
We can also say 2 mol of water (H20)
contains 1 mol of oxygen (02).
molesofO2 =6.7molH20.
1molof O2\ 2 molo of H2 O = 3.35molofO?
Calculate the proper number of significant digits, the density of a 23.23g box occupying 26.5 mL.
Answers
Answer:
0.877 mL
Explanation:
The box's density would be the ratio of the mass of the box and its volume
which is, (23.23/26.5) mL
or, 0.8766 mL
We must round this down to 3 significant figures,
which will be 0.877 mL
What else should the second group of researchers measure to test the hypothesis
Answers
Answer:
Testing of thesis testing is used to determine the plausibility of a thesis.
The test assess the plausibility of the thesis of a sample data.
It occurs through different way, the first step is used to test the state the two suppositions.
In the coming step evaluation of data, third step include carry out plan and dissect the data.
The final step is to dissect the results of the data, it can moreover accept the thesis and reject the null thesis.
Learn further about thesis, then
brainly.com/question/17173491?
Which statement below can NOT
be used when describing volume?
A. Volume is the amount of space that an object
occupies.
B. The volume of an object is the same as the
weight of that object.
C. Volume can be measured in cubic
centimeters.
D. Volume is calculated using a formula: V = 1x w
xh.
Answers
Answer:
the answer is B:
Explanation:
I hope it help.
Sulfur is composed of three isotopes: 32S, 33S, and 34S. The atomic masses of these isotopes are given below. 32S: 31.97207 amu 33S: 32.97146 amu 34S: 33.96786 amu The abundance of 34S is 4.22%. Given the average atomic mass of sulfur (32.07 amu), determine the abundance of 32S to two decimal places.
Answers
Answer:
Abundance of 32S is 94.41%
Explanation:
The average atomic mass is defined as the sum of the atomic masses of each isotope times its abundance:
Average atomic mass = ∑ Atomic mass istope*Abundance
For the sulfur:
32.07amu = 31.97207X + 32.97146Y + 33.96786*0.0422 (1)
Where X is abundance of 32S and Y abundance of 33S
Also we can write:
1 = X + Y + 0.0422 (2)
0.9578 - X = Y
Because the sum of the abundances = 1
Replacing (2) in (1):
32.07amu = 31.97207X + 32.97146(0.9578 - X) + 33.96786*0.0422
32.07 = 31.97207X + 31.58006 - 32.97146X + 1.43344
-0.9435 = -0.99939X
0.9441 =X
In percentage, abundance of 32S is 94.41%
Calculate the pOH if the [OH-] concentration is 5.9 x 10_, M? Is the solution ACIDIC, BASIC, or NEUTRAL?
Answers
If the [OH-] concentration is 5.9 x 10^(-M), the pOH of the solution is approximately -4.77 and the solution is basic.
To calculate the pOH of a solution, we can use the formula:
pOH = -log[OH-]
Given that the [OH-] concentration is 5.9 x 10^(-M), we can substitute this value into the formula:
pOH = -log(5.9 x 10^(-M))
Calculating this expression, we find:
pOH = -log(5.9 x 10^(-M))
pOH ≈ -log(5.9) + (-log(10^(-M)))
Since log(10^(-M)) is equal to -M, the equation simplifies to:
pOH ≈ -log(5.9) - M
Now, we need the value of M (the exponent) to calculate the exact pOH value. It appears that the value of M is missing in the given information. However, assuming M is a positive value, we can continue the calculation.
If we consider M = 6, for instance, the equation becomes:
pOH ≈ -log(5.9) - 6
Now, we can evaluate the expression:
pOH ≈ 1.23 - 6
pOH ≈ -4.77
Therefore, if the [OH-] concentration is 5.9 x 10^(-M), the pOH of the solution is approximately -4.77.
To determine whether the solution is acidic, basic, or neutral, we can use the relationship between pH and pOH. The sum of the pH and pOH of a solution at 25°C is always equal to 14.
Since pOH = -4.77, the pH would be:
pH = 14 - pOH
pH ≈ 14 - (-4.77)
pH ≈ 18.77
A solution with a pH above 7 is considered basic. In this case, the calculated pH is greater than 7. Therefore, the solution is basic.
for more questions on pOH
https://brainly.com/question/1420583
#SPJ11
how many molecules are present in 7 moles of h2o
Answers
Answer:
9moles of h20 hndi parin ako sure
discuss whether the law of conservation of mass offers an explanation for observed relationships.
Answers
Answer:
Examine these two MSDS from different manufacturers for sodium hydroxide (NaOH). Compare and contrast the following aspects: chemical names, chemical properties, order of components, health hazards, and proper disposal. Click on each of the links to examine two MSDS reports from different sources.
Explanation: