Pls Help ASAP! 2 grams of potassium (K) reacts with 5 grams of Oxygen (O). According to the Law of Conservation of Mass, how many grams of potassium oxide (K2O) will be produced?
O 10 grams
O 7 grams
O 2 grams
O 5 grams
Answers
Taking into account the reaction stoichiometry, 2.4041 grams of K₂O can be produced from 2 grams of K and 5 grams of O₂.
Reaction stoichiometryIn first place, the balanced reaction is:
4 K + O₂ → 2 K₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
K: 4 molesO₂: 1 mole K₂O: 2 molesThe molar mass of the compounds is:
K: 39.1 g/moleO₂: 32 g/moleK₂O: 94.2 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
K: 4 moles ×39.1 g/mole= 156.4 gramsO₂: 1 mole ×32 g/mole= 32 gramsK₂O: 2 moles ×94.2 g/mole= 188.4 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 32 grams of O₂ reacts with 156.4 grams of K, 5 grams of O₂ reacts with how much mass of K?
mass of K= (156.4 grams of K× 5 grams of O₂)÷ 32 grams of O₂
mass of K= 24.4375 grams
But 24.4375 grams of K are not available, 2 grams are available. Since you have less mass than you need to react with 5 grams of O₂, K will be the limiting reagent.
Mass of K₂O formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 156.4 grams of K form 188 grams of K₂O, 2 grams of K form how much mass of K₂O?
mass of K₂O= (2 grams of K× 188 grams of K₂O)÷ 156.4 grams of K
mass of K₂O= 2.4041 grams
Then, 2.4041 grams of K₂O can be produced from 2 grams of K and 5 grams of O₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Answer:
(Question) What does the Law of Conservation of Mass state?
(Answer) The total mass of all of the reactants prior to a chemical reaction must equal the total mass of all the products after the reaction.
(Question) If the mass of elements before a chemical reaction is 30 grams, after the chemical reaction, the mass will be __.
(Answer) 30 grams
(Question) 78 g of potassium (K) react with 71 g of chlorine (Cl) to produce potassium chloride. According to the Law of Conservation of Mass, what is the mass of the product (2KCl)?
(Answer) 149 g
(Question) 2 grams of potassium (K) reacts with 5 grams of Oxygen (O). According to the Law of Conservation of Mass, how many grams of potassium oxide (K2O) will be produced?
(Answer) 7
(Question) Which of the following equations demonstrates the Law of Conservation of Mass?
(Answer) CH4+O2→C+2H2O
Explanation:
just finished the quick check. hope this helps UwU
Related Questions
Consider the reaction
4Fe + 30, - 2FeO,
Round to 2 decimals for your response. If 540g of iron react with 400g of oxygen gas, which will be the limiting reactant? 540g of
iron can produce
type your answer... moles of iron oxide. And, 400g of oxygen gas can produce type your answer...
moles of iron oxide. choose your answer...
will be the limiting reactant.

Answers
For Iron :
n Fe = m Fe / Mr Fe
n Fe = 540 / 55.85
n Fe = 9.67 mol
n Fe₂O₃ = (2/4) • 9.67
n Fe₂O₃ = 4.83 mol
For Oxygen :
n O₂ = m O₂ / Mr O₂
n O₂ = 400 / 32
n O₂ = 12.5 mol
n Fe₂O₃ = (2/3) • 12.5
n Fe₂O₃ = 8.33 mol
Fe is the limiting reactant
Be sure to answer all parts. Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? When drawing alkene substituents, remember that it is preferable to draw them as regular lines than as dashes and wedges. a. untitled draw structure ...
Answers
Answer:
hello your question is incomplete attached below is the complete question and answer
answer: attached below
Explanation:
A) attached below
B) attached below


The mixture of kaolin and petunse, when fired at a high temperature, is known as ____________ .
Answers
The correct answer for this question is 'Porcelain'
Firstly, let's have a look about what kaolin and petunse are,
Kaolin:
Kaolin, commonly known as China clay, is a soft white clay that is used to make a variety of items, including paper, rubber, paint, and many other goods. It is a necessary component in the production of China and porcelain.
Petunse:
Rocks with a high concentration of mica and feldspathic minerals, which have been utilized as one of the components in the production of Chinese porcelain, are known historically in Chinese as petunse. It is generally comparable to China stone in composition and usage.
What is porcelain?
Ceramic ingredients such as kaolinite are often heated to temperatures between 1,200 and 1,400 °C (2,200 and 2,600 °F) in a kiln to create porcelain.
To know more about Porcelain, click here:
https://brainly.com/question/14653842
#SPJ4
1. Three fluids are poured from a beaker onto a lab table. Fluid A hits the table in 10 seconds, fluid B in 12 seconds, fluid C in 4 seconds, and fluid D
in 15 seconds. Which fluid has the highest viscosity?
O a fluid A
O b. fluid B
O c. fluid c
O d. fluid D
Answers
Answer:
fluid D
Explanation:
Fluid D has the highest viscosity because it is more difficult for it to move which makes it hit the table at 15 seconds
while fluid C has the lowest Viscocity which makes it move easily and it hit the table fastest
6. What type of energy is the result of water and hot matter interacting?
Answers
Boiling water on a stove is an example of thermal energy. Thermal energy is produced when the atoms and molecules in a substance vibrate faster due to a rise in temperature.
where in the air-conditioning system is the refrigerant a low-pressure gas
Answers
In an air-conditioning system, the refrigerant undergoes a cycle of evaporation and condensation to cool the air. The refrigerant starts as a low-pressure gas in the evaporator coil, where it absorbs heat from the surrounding air and evaporates into a low-pressure gas. This evaporation process cools the air as well. The evaporator coil plays a crucial role in this system by facilitating the evaporation of the refrigerant and cooling the air.
On the other hand, the refrigerant transitions from a high-pressure gas to a high-pressure liquid in the condenser. The condenser is responsible for releasing the heat absorbed by the refrigerant in the evaporator to the surrounding air. As the refrigerant condenses into a high-pressure liquid, it releases heat, allowing it to transfer the heat from the inside of a building to the outside.
This cycle of evaporation in the evaporator and condensation in the condenser enables the refrigerant to remove heat from the indoor space and release it outside. By continuously circulating the refrigerant and repeating this process, an air-conditioning system can cool the air inside a building and maintain a comfortable temperature.
To know more about evaporator and condensation
https://brainly.com/question/956180
#SPJ11
which one of the following is different from the others A .HCl B. HF C. HBr D. HI
Answers
Answer:
D.HI
Explanation:
because this is the most different
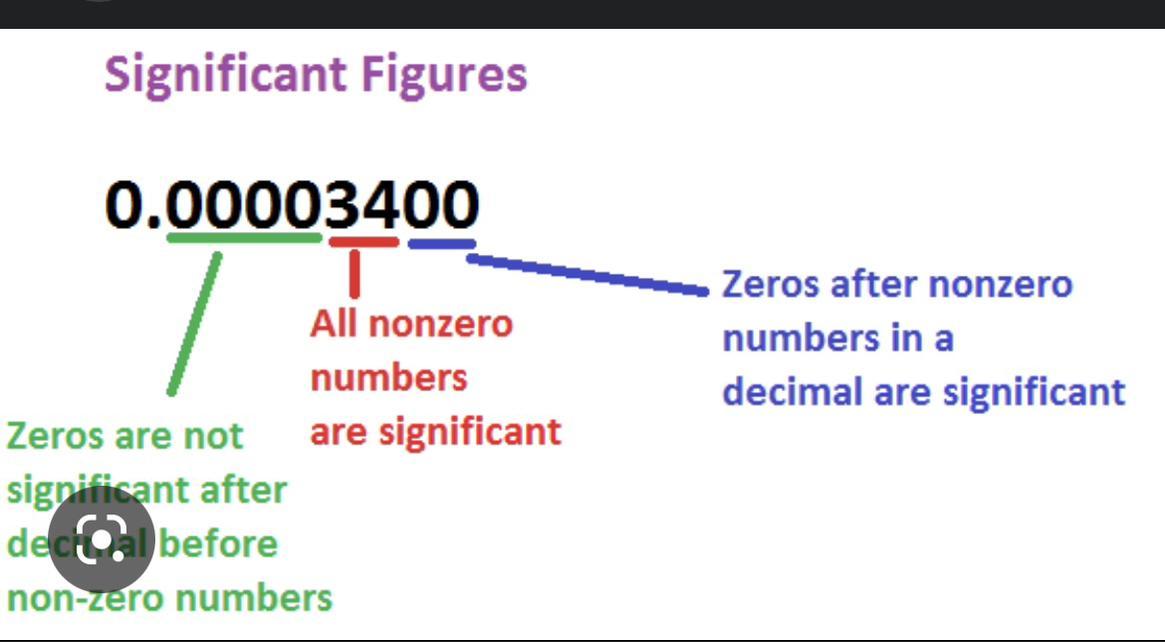
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
select the classification for the following reaction. koh(aq) + hcl(aq) → kcl(aq) + h2o(l) A.None of these choices is correct. B..acid-base C.precipitation D.combination E.redox
Answers
The reaction is : KOH + HCl ---> KCl + H₂O is double displacement reaction and it is also the neutralization reaction . So, the correct option is B) acid - base.
The reaction is given below :
KOH + HCl ---> KCl + H₂O
This reaction is the neutralization reaction as well as the double displacement reaction. The neutralization reaction is the reaction in which the acid and base react and produces the salt and the water. This is also called as the acid base reaction.
The double displacement reaction is the reaction in which the reactant ions exchange places and form the new product.
To learn more about double displacement here
https://brainly.com/question/29307794
#SPJ4
How do I find the X in 5g of Na2CO3.XH2O
Answers
The Value of X in 5g of Na2CO3.XH2O is 2.
To find the value of X in the compound Na2CO3·XH2O, we need to determine the number of water molecules (H2O) associated with each formula unit of Na2CO3. This can be done by performing a simple experiment called "dehydration."
To begin, we measure 5 grams of Na2CO3 and heat it to a high temperature. As heat is applied, the water molecules associated with the compound will evaporate, leaving behind an anhydrous form of Na2CO3. After heating, we measure the remaining mass of the compound.
Let's assume that after heating, the mass of the anhydrous Na2CO3 is found to be 3 grams. This means that 2 grams of water (H2O) were lost during the dehydration process.
To determine the value of X, we need to calculate the number of moles for both Na2CO3 and H2O. The molar mass of Na2CO3 is 106 g/mol, while the molar mass of H2O is 18 g/mol.
The number of moles of Na2CO3 can be calculated using the formula: moles = mass / molar mass. Thus, moles of Na2CO3 = 3 g / 106 g/mol = 0.0283 mol.
Since the ratio between Na2CO3 and H2O in the compound is 1: X, we can conclude that 0.0283 mol of Na2CO3 corresponds to 2 grams of water (H2O).
Now, we can set up a proportion to find the value of X:
0.0283 mol Na2CO3 / 2 g H2O = 1 mol Na2CO3 / X g H2O
Simplifying the equation, we find:
X = (2 g H2O * 0.0283 mol Na2CO3) / (0.0283 mol Na2CO3)
X = 2 g
Therefore, the value of X in the compound Na2CO3·XH2O is 2.
Know more about the compound here:
https://brainly.com/question/28781679
#SPJ8
the temperature of ice is lower than the temperature of water. the temperature of water and ice are the same. the density of ice is less than the density of water. the density of ice is greater than the density of water?
Answers
Answer:
When water freezes, water molecules form a crystalline structure maintained by hydrogen bonding. Solid water, or ice, is less dense than liquid water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart, which lowers the density.
A study was conducted of 90 adult male patients following a new treatment for congestive heart failure. One of the variables measured on the patients was the increase in exercise capacity (in minutes) over a 4-week treatment period. The previous treatment regime had produced an average increase of μ=2 minutes. The researchers wanted to evaluate whether the new treatment had increased the value of μ in comparison to the previous treatment. The data yielded y(bar)=2.17 and s=1.05.
(a) if the actual value of mu is 2.1 and alpha is reduced from 0.05 to 0.01, what would be the effect on the power curve?
(b) If the sample size is reduced from 90 to 50, what would be the effect on the power curve?
Answers
a. Decreasing alpha from 0.05 to 0.01 makes the significance level more stringent. You will be less likely to reject the null hypothesis, even when it's false. This increases the probability of a Type II error, thus potentially reducing the power of the test. The power curve will shift to the left.
b. If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
What more should you know about decreasing the alpha and the power curve?The power curve is a graph that shows the probability of rejecting the null hypothesis as a function of the true value of the mean.
In the given scenarios of this study, Reducing the significance level and reducing the sample size will shift the power curve to the left, indicating a decrease in the statistical power of the test.
The power of a statistical test is the probability that it correctly rejects the null hypothesis when the alternative hypothesis is true.
a) Reducing alpha from 0.05 to 0.01 means that we are more stringent in our assessment of whether the new treatment is effective.
This will result in a decrease in the power of the test, meaning that it is less likely that we will be able to detect a difference between the new treatment and the previous treatment.
b) If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
This is because a smaller sample size decreases the power of the test. A larger sample size provides more information and thus makes it more likely to correctly reject the null hypothesis when the alternative hypothesis is true.
Therefore, by reducing the sample size, you are decreasing the likelihood of detecting a true effect if one exists, thus reducing the power of the test.
Find more exercises on alpha level in a study;
https://brainly.com/question/6372035
#SPJ4
does magnesium or strontium have a greater ionization energy
Answers
Magnesium has a lower ionization energy than Strontium. The ionization energy of magnesium is 738 kJ/mol while that of Strontium is 549 kJ/mol. Therefore, magnesium is easier to ionize compared to Strontium.
Magnesium has a lower ionization energy compared to Strontium. Magnesium and strontium both belong to group 2 (alkaline earth metals) in the periodic table. Their atomic numbers are 12 and 38, respectively. When a metal atom loses one or more electrons to form a positively charged ion, the energy required is called the ionization energy. Ionization energy increases from left to right and from bottom to top across the periodic table. It means that as one moves from left to right in a periodic table, the ionization energy of the element increases. Similarly, as one moves from bottom to top in a group, the ionization energy of the element increases. Magnesium has a lower ionization energy than Strontium. The ionization energy of magnesium is 738 kJ/mol while that of Strontium is 549 kJ/mol. Therefore, magnesium is easier to ionize compared to Strontium.
To know more about ionization energy visit :
brainly.com/question/28385102
#SPJ11
SOMEONE HELP ME PLEASE
Enter answer for number 1
2:00 minutes
8:00 minutes

Answers
Based on factors affecting reaction rate , the time for colouring at cold temperature 5°C will be 8.00 minutes.
How does temperature affect rate of a reaction?The rate of a reaction increases with increase in temperature.
The increase in reaction rate is due to the increase in the kinetic energy of the molecules reacting.
A low temperature lowers reaction rate.
Thus, at cold temperature 5°C, the time for food colouring will be less than at room temperature.
Therefore, the time for colouring at cold temperature 5°C will be 8.00 minutes.
Learn more about reaction rate at: https://brainly.com/question/7578129
Please help me no links
if good answer you get brainliest

Answers
Answer:
plutonic rocks are formed when magma cools and solidifies underground This shows us if the rock is plutonic or volcanic. When magma cools underground, it cools super slow and when lava cools above ground, it cools faster. When magma and lava cool, mineral crystals start to form in the molten rock.
Explanation:
i hope this is correct
Explain anaerobic respiration with yeast in your own words
Answers
Answer:
Is breaking down of food substances in the abscence of Oxygen
Compare and contrast a thermodynamically stable compound and a kinetically stable compound at room temperature with respect to overall energy change, spontaneity with which decomposition occurs, and reaction rate.
Answers
Answer:
"im different"
Explanation:
in addition to 2-butanone there is (are) ____ more ketones with the formula c4h8o.none, one, two, three?
what is it (are they)?
Answers
There are two more ketones with the formula C4H8O, and they are 3-pentanone and 2-pentanone. Ketones are a type of organic compound that have a carbonyl group attached to two alkyl or aryl groups. They are commonly used in the production of solvents, plastics, and other chemicals.
2-Butanone, also known as methyl ethyl ketone, is a colorless liquid with a sweet, pungent odor. It is widely used as a solvent for various materials such as resins, coatings, and adhesives. In addition to its industrial applications, 2-butanone is also used in some consumer products such as nail polish remover and paint thinner.
3-Pentanone and 2-pentanone, on the other hand, are both colorless liquids with a similar odor to acetone. They are also used as solvents and in the production of other chemicals. However, they are less commonly used than 2-butanone.
In conclusion, there are three ketones with the formula C4H8O: 2-butanone, 3-pentanone, and 2-pentanone. While 2-butanone is the most widely used of the three, all three compounds have important industrial applications.
For more information on ketone see:
https://brainly.com/question/4439718
#SPJ11
What is Nuclear fusion
Answers
Answer:
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons).
in part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample of household bleach is completely reacted with ki(s). the resulting solution is then titrated with 0.150 m nas2o3 solution. 0.890 ml of the solution is required to reach the colorless endpoint. what is the mass percent of naocl (mm
Answers
There is 5.8 % of NaOCl in the sample.
The oxidation of hypochlorite is the first process, which goes as follows:
OCl^- + 2I^- + 2H^+ -----> I2 + Cl2 + H2O
Iodine and sodium thiosulfate react in the second stage as follows:
I2 + 2NaS2O3 -----> Na2S4O6 + 2NaI
Thiosulfate moles = 0.150 M * 0.890/1000 L = 1.335 * 10-4 moles.
Since 2 molecules of thiosulfate react with 1 mole of I2,
1.335 * 10-4 moles of thiosulfate and x moles of I2 react.
x = 1.335 * 10-4 moles/2 moles times one mole.
Iodine equivalent to 6.675 x 10-5 moles
6.675 * 10-5 moles of hypochlorite were reacted because 1 mole of hypochlorite yields 1 mole of iodine.
Therefore, the mass of NaOCl is equal to 4.97 * 10-3 g (6.675 * 10-5 moles * 74.44 g/mole).
5.8% of NaOCl is equal to 4.97 * 10-3 g/0.0854 g * 100/1.
NaOCl makes up 5.8% of the sample.
To know more about bleach refer to https://brainly.com/question/13572752
#SPJ4
What is the formula unit for Pb4+ and O?
Answers
Answer:
Pb O2
Explanation:
What is the formula unit for a compound made from Pb 4 and oxygen?
For each substance, designate whether you expect it to be most soluble in methanol (CH
3
OH), hexane (C
6
H
14
) or acetone (CH
3
COCH
3
). a. NaCl b. I
2
c. CH
3
OCH
3
d. water
Answers
Solubility can be defined as the capacity of a substance to dissolve in another substance. The type of solvent influences the solubility of a compound. The solubility of a substance is often determined by the polarity of the solvent and the solute.
The substance will dissolve if the intermolecular forces are identical between the solvent and the solute. Conversely, if the intermolecular forces are different between the solvent and the solute, the solute will not dissolve.For each substance, designate whether you expect it to be most soluble in methanol (CH3OH), hexane (C6H14), or acetone NaClNaCl is a salt that is readily soluble in water.
This is because NaCl has a high ionic character, and both methanol and acetone are polar solvents, whereas hexane is a nonpolar solvent.b. I2I2 is nonpolar and dissolves easily in nonpolar solvents like hexane. It is, however, insoluble in polar solvents such as methanol and acetone. In this case, intermolecular forces are the driving force for solubility, and I2 lacks the ability to form hydrogen bonds.CH3OCH3 Dimethyl ether, also known as CH3OCH3, is a polar aprotic solvent. Dimethyl ether can dissolve polar and nonpolar solutes to some degree, so it is most likely soluble in all three solvents.
To know more about Solubility visit :
https://brainly.com/question/31493083
#SPJ11
For the bonds N-O and O-F what atoms carry the δ+ and δ-?
Answers
Answer:
In N-O, the delta + is on N and delta - on O while in O-F the delta + is on O and delta - is on F.
Explanation:
Hope this helped! :)
Happy Early Valentine's Day!
Brainliest, Please!
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
What are isotopes?
A.) Atoms of the same element that have a different number of protons
B.) Atoms of the same element that have a different number of neutrons
C.) Atoms of different elements that have the same number of neutrons
D.) Atoms that are unstable
Answers
Answer:
B
Explanation:
Answer:
B. ATOMS OF THE SAME ELEMENT THAT HAVE A DIFFERENT NUMBER OF NUETRONS
Explanation:
Each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element.
How many valence electrons are in an atom of each element in group 17 in the periodic table?.
Answers
There are seven valence electrons present in an atom of each element in group 17 of the periodic table.
Valence electrons are the electrons present in the outermost shell of an atom that are involved in chemical reactions. The number of valence electrons an atom has depends on its group number in the periodic table.Elements in group 17 are known as the halogens. They include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements all have seven electrons in their outermost shell, making them have seven valence electrons. This means that they are one electron short of having a full outer shell of eight electrons, which is the ideal electron configuration known as the octet rule.
The valence electrons in an atom of group 17 elements are responsible for their reactivity. They tend to form ionic bonds with metals to achieve a full outer shell, resulting in the formation of compounds known as halides. For example, chlorine can form an ionic bond with sodium to create sodium chloride (NaCl), which is table salt.
To know more about atom visit:
https://brainly.com/question/1566330
#SPJ11
Radioisotopes have been used to determine the age of all of the following EXCEPT:
a)brandy
b) cloth
c) living plants
d) wood
Answers
The answer is a) brandy. Radioisotopes have been used to determine the age of various materials through radiometric dating techniques.
These techniques rely on measuring the decay of radioactive isotopes present in the material to estimate its age. For example, carbon-14 dating is commonly used to determine the age of organic materials such as cloth, living plants, and wood. By contrast, brandy, being a distilled alcoholic beverage, does not contain organic material that can be used for radiometric dating. Instead, other methods, such as historical documentation or chemical analysis, are typically employed to determine the age of brandy.
To learn more about chemical click here:brainly.com/question/29240183
#SPJ11
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
formula of sodium chloride, sodium trioxocarbonate (IV), aluminum oxide, ammonium sulphide, zinc hydroxide, zinc tetraoxosulphate (IV), sodium tetraoxosulphate (VI), trioxonitrate (V) acid, water
Answers
Answer:
The correct answer is Na2CO3. 10H2O
AH for the reaction:
SO
2 (g) → S (s) + O2 (g)
Answers
Answer:
the ans is wrong