PLEASEEE HELP MEEEE!!! How many grams of iron (III) oxide will be produced if 4300 kJ of heat energy is released?
4 Fe+ 3 O2 → 2 Fe2O3
ΔH = -1652 kJ
Answers
Answer: 652.8 g of iron (III) oxide produced.
Explanation:
To calculate the amount of iron (III) oxide produced, we use the enthalpy change of the reaction to determine the amount of energy released and convert it to moles of Fe2O3 produced. Then, we multiply by the molar mass of Fe2O3 to obtain the mass of Fe2O3 produced. Using these calculations, we get 652.8 g of iron (III) oxide produced.
580.84 grams of iron (III) oxide will be produced when 4300 kJ of heat energy is released.
Given:
Enthalpy change (∆H) value: ∆H = -1652 kJ
Amount of heat energy released: 4300 kJ
From the balanced equation:
4Fe + 3O₂ → 2 Fe₂O₃
The molar ratio between Fe₂O₃ and ∆H is 2:1652 kJ.
To find the molar amount of Fe₂O₃ produced, the following calculation:
\(4300 \times \frac{2}{1652}\) = 5.20 mol Fe₂O₃
To convert this into grams, it is required to multiply the molar amount by the molar mass of Fe₂O₃:
5.20 × 2 × 55.85 = 580.84 g
Therefore, 580.84 grams of iron (III) oxide will be produced when 4300 kJ of heat energy is released.
Learn more about heat energy, here:
https://brainly.com/question/1495272
#SPJ2
Related Questions
Stanley miller's experiments were significant because he demonstrated that __________.
Answers
He demonstrated that a large variety of organic compounds could be spontaneously synthesized from components in Earth's primitive atmosphere
What is Stanley miller's experiments ?Scientist Stanley Miller conducted an experiment in 1953 that might shed light on what happened on early Earth billions of years ago. A flask containing a chemical mixture of methane, ammonia, hydrogen, and water was subjected to an electrical charge. Amino acids and other chemical molecules were produced as a result.
In the prebiotic soup of Earth, the study found a pathway from simple to complex chemicals. Peptides could have been created from amino acids more than 4 billion years ago. The proteins and enzymes required for life's biochemistry as we know it today may have ultimately evolved from these peptides.Learn more about Stanley miller's experiments here:
https://brainly.com/question/22515309
#SPJ4
after takeoff you encounter a temperature inversion you should expect
Answers
When encountering a temperature inversion after takeoff, you should expect changes in atmospheric conditions, such as a decrease in temperature with increasing altitude instead of the usual temperature increase.
This can lead to challenges in aircraft performance and may require adjustments in flight operations. A temperature inversion refers to a deviation from the typical atmospheric temperature pattern where temperature decreases with increasing altitude. In a standard atmosphere, the temperature usually decreases by about 2 degrees Celsius per 1,000 feet of altitude gain. However, in a temperature inversion, there is a reversal of this pattern, resulting in a layer of warmer air above cooler air.
Encountering a temperature inversion after takeoff can have several implications for aircraft operations. Firstly, the inversion layer acts as a boundary that can affect the performance of the aircraft. It can cause changes in air density, which may result in alterations to lift and drag forces. These changes can impact aircraft stability, climb performance, and fuel efficiency.
Secondly, a temperature inversion can lead to the formation of fog or low-level clouds within the inversion layer. Moisture present in the cooler air below the inversion may condense as it comes into contact with the warmer air above. This can reduce visibility and pose challenges for navigation.
In such situations, pilots need to be aware of the temperature inversion and its effects on aircraft performance. They may need to adjust their flight operations, such as modifying climb rates or considering alternate routes to avoid adverse conditions. Communicating with air traffic control and staying informed about weather updates can help pilots make informed decisions and ensure a safe flight.
To learn more about temperature inversion refer:
https://brainly.com/question/3083526
#SPJ11
physical and chemical proportys
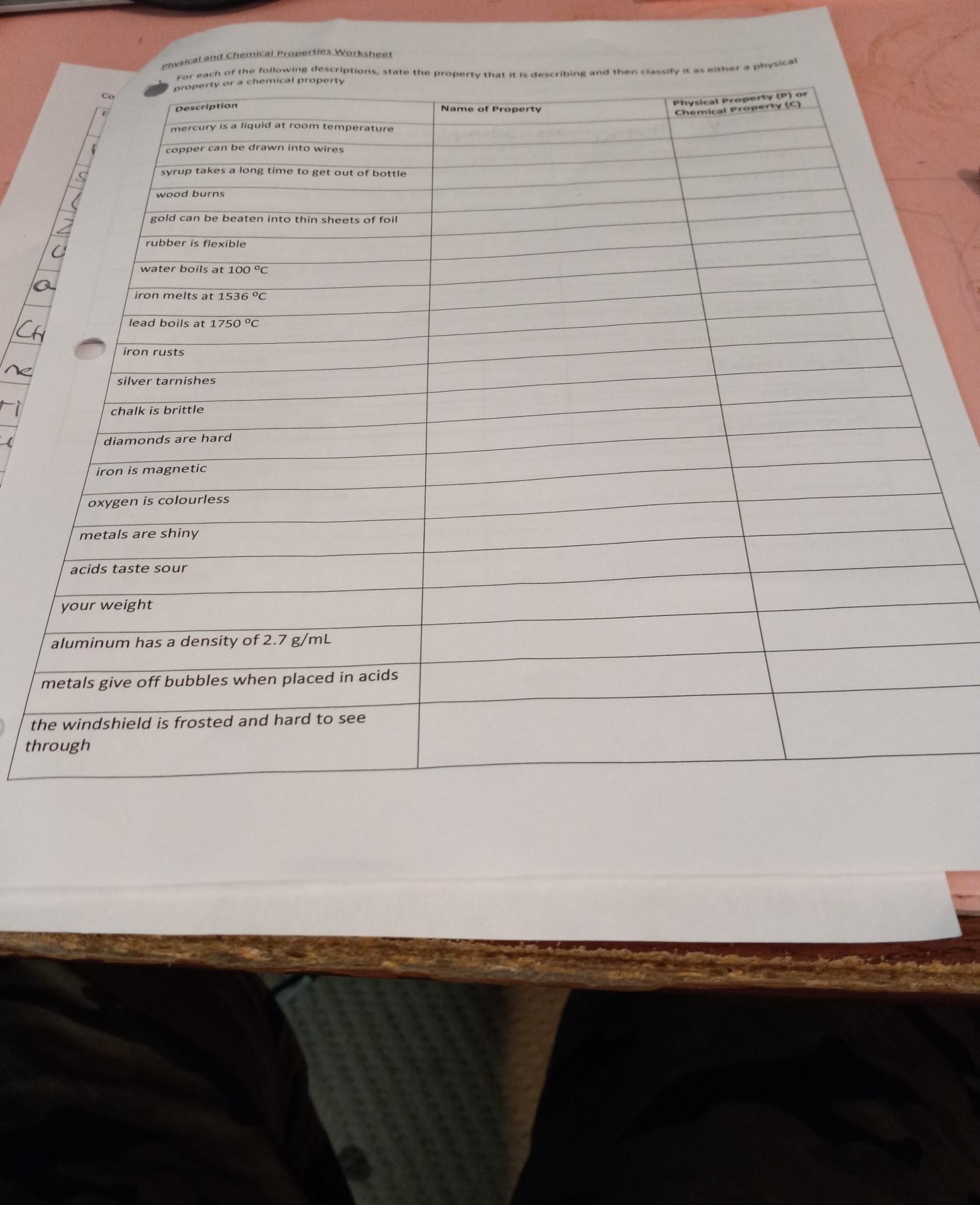
Answers
The physical and the chemical properties of matter are listed below.
What are the examples of physical and chemical properties?While the entire page is not clear, I have an idea of what you are trying to ask and I would show you the physical and the chemical properties of matter.
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition. Examples of physical properties include:
Density
Color
Boiling point
Melting point
Hardness
Solubility
Electrical conductivity
Optical properties (such as refractive index)
Chemical properties are characteristics of a substance that describe its ability to undergo chemical reactions and form new substances. Examples of chemical properties include:
Reactivity with other substances
Combustibility
Oxidation state
Acidity or basicity (pH)
Toxicity
Flammability
Learn more about matter:https://brainly.com/question/28487167
#SPJ1
What does Fe(NO3)2 + Cu stands for?
Answers
Copper nitrate is the oxidizing agent as it gets reduced from +2 to 0 oxidization State and oxidize Fe from 0 to +2 state.
A covalent chemical bond is one in which:_____.a) outer-shell electrons of one atom are transferred to the inner electron shells of another atom.b) protons or neutrons are shared by two atoms so as to satisfy the requirements of both.c) the inner-shell electrons of one atom are transferred to the outer shell of another atom.d) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.e) outer-shell electrons are shared by two atoms so as to satisfactorily fill the outer electron shells of both.f) an electron occupies a hybrid orbital located between the nuclei of two atoms.
Answers
Answer:
A.outer-shell electrons of one atom are transferred to the inner electron shells of another atom.
Explanation:
In covalent chemical bond, outer shell share pair of electrons to fill both atoms outer shell. Covalent bond can be polar and non-polar. Hydrogen and carbon bonding is example of non-polar bonds; while hydrogen bonding with chlorine is polar covalent bond example
50 g of NaF is added to 100 mL of water to form a solution. What is the concentration of the solution?
Answers
no ls eb vb vj lfn nbf, l mgñ rlr o
A man drops a baseball off of the top of the Empire State Building. If the action force is the Earth pulling on the ball, then what is the
reaction force?
OA.
the acceleration of the ball
ОВ.
the ball pulling on the Earth
OC.
air resistance acting on the ball
OD
the ground pushing on the ball
Answers
During a chemical reaction, a new ___________ is formed. what is formed
Answers
Why is acid added to water first
Answers
Answer: because acid and water react in a vigorous exothermic reaction, releasing heat, sometimes boiling the liquid.
what is a well-tested, explanation that unifies a broad range of observations and hypotheses
Answers
Following the laser stimulation of the photostimulable phosphor, the excited electrons are ____.
Answers
Photostimulation is a process that includes the absorption of electromagnetic radiation, particularly light, by chromophores or fluorophores to initiate a cellular response. Light-induced physiological responses include vision, plant growth, and photomorphogenesis, among others.
Following the laser stimulation of the photostimulable phosphor, the excited electrons are trapped in the conduction band.What is photostimulation?
Photostimulation is a process that includes the absorption of electromagnetic radiation, particularly light, by chromophores or fluorophores to initiate a cellular response. Light-induced physiological responses include vision, plant growth, and photomorphogenesis, among others.
Laser stimulation
Laser stimulation is the use of laser light to control cellular activity, frequently using ion channels or opsins that have been genetically modified to respond to light. While traditional electrophysiology techniques rely on electrical stimulation of cells, optogenetics employs light as a means of non-invasive and selective manipulation of neural systems or tissues.
Photostimulable
Photostimulable refers to the phosphor's ability to release energy after excitation by light, which allows for energy transfer to the electrons that are trapped in the conduction band. In conclusion, we can say that following the laser stimulation of the photostimulable phosphor, the excited electrons are trapped in the conduction band.
To know more about electromagnetic radiation visit:
https://brainly.com/question/29646884
#SPJ11
A sample of dinitrogen tetroxide (N204) gas occupies 144 L at STP. How many moles of nitrogen atoms are present in this sample?
Answers
The number of moles of nitrogen atoms present in the sample is: 12.86 mol of nitrogen atoms
At STP (Standard Temperature and Pressure), the temperature is 273 K and the pressure is 1 atm. The molar volume of any ideal gas at STP is 22.4 L/mol. Therefore, the number of moles of N204 gas present in the sample can be calculated as:
n = V/VM
where n is the number of moles, V is the volume of the gas, and VM is the molar volume of the gas at STP.
n = 144 L / 22.4 L/mol = 6.43 mol
Since dinitrogen tetroxide contains two nitrogen atoms per molecule, the number of moles of nitrogen atoms present in the sample is:
2 x 6.43 mol = 12.86 mol of nitrogen atoms.
To know more about atoms click here:
brainly.com/question/29695801
#SPJ4
6. Perform the following calculations and report each answer to the correct number of significant figures: a. 162.1 g + 38.73 g + 1.554 g
b. 21.9 m + 6.34 m + 157 m
c. 0.004 dm + 0.12508 dm
d. 2.0 L + 2.4L + 2.51L
e. .025 mol + .0267 mol + .00287 mol
f. 9.88 s-7.2 s
g. 44.7 kg - 2.7 kg
h. 20 L - 20.0 L i. 2.89g - 3.00g
j. 8.894 mL - 9.23 mL
Answers
Answer:
Explanation:
a) 202.4 g
b) 185.24 m
c) 0.13 dm
d) 6.91 L
e)0.0546 mol
f) 2.7 s
g) 42kg
h) 0
i) -0.11 g
j) -0.34 mL
Which of the following is a molecular compound that produces H+ ions when dissolved in water?
a. H2SO4
b. Hg(NO3)2
c. CH4
d. NaC2H3O2
Answers
Option - a is correct answer, H₂SO₄ is a molecular compound that produces H⁺ ions when dissolved in water.
What constitutes a molecular compound example?Molecule-shaped inorganic substances are known as molecular compounds. Examples include common compounds like water (H₂O) and carbon dioxide (CO₂). When compared to ionic compounds like sodium chloride, these compounds are very different (NaCl).
Molecules that have a formula that reflects the number of atoms actually bound together in the molecule make up a molecular compound. The bonds between the atoms are joined to form a distinct shape, which is determined by the bond lengths and angles.
Acid produces proton when there is water present.
H₂SO₄ + H₂O → H₃O⁺ + HSO₄⁻
To know more about molecular compound visit:
https://brainly.com/question/23088724
#SPJ1
what is the reason of the gas pressure.
Answers
Answer:
The rapid motion and collisions of molecules with the walls of the container causes pressure (force on a unit area). Pressure is proportional to the number of molecular collisions and the force of the collisions in a particular area. The more collisions of gas molecules with the walls, the higher the pressure
What is the percent by volume of ethanol in gasohol when 95 ml of ethanol is added to sufficient gasoline to make 1.0 l of gasohol?
Answers
The percentage of volume of ethanol is 9.5%.
What is volume by volume percentage?Volume/volume percentage (v/v% or percent v/v) is a unit used to express how much of a material is present in a solution. It is defined as the volume of the solute divided by the sum of the volumes of the solution, multiplied by one hundred. Examples: The average alcohol concentration (v/v%) of wine is 12 percent.
The formula used to get the volume of ethanol in percentages is
Percent of volume of ethanol = (volume of solute / total volume) * 100
Percent volume of Ethanol = (volume of ethanol / total volume) * 100
= 95mL / 1000mL * 100
= 9.5%
to know more go to - https://brainly.com/question/14589084
#SPJ4
(4.5x10x10x10x10x10)÷(300)
Answers
Answer:
Explanation:
4.5x10x10x10x10x10 can be written as 4.5^5, by the way.
The sum of 4.5^5 is 450,000.
450,000 divided by 300 is 1500.
Hope this helps!
Explanation:
4.5×10×10×10×+0×10=4500000
450000÷300
remove zeros
4500÷3
=1500
Determine the number of calories required for 47.5g of Al to go from 25 to 62. The specific heat of Al is 0.900 J/g C.
Answers
The number of calories required for 47.5g of Al to go from 25 to 62 is 381.1 calories.
What is Calories?Calories are a unit of energy. Specifically, the calorie (cal) is the amount of energy required to raise the temperature of 1 gram of water by 1 degree Celsius. The calorie is commonly used in nutrition to describe the amount of energy provided by food. In scientific contexts, the kilocalorie (kcal), which is equal to 1000 calories, is often used as a unit of energy.
To solve this problem, we will use the following formula:
q = m * c * ΔT
Where:
q = heat energy required (in calories)
m = mass of the substance (in grams)
c = specific heat capacity (in J/g C)
ΔT = change in temperature (in C)
Substituting the given values, we get:
q = 47.5 g * 0.900 J/g C * (62 C - 25 C)
q = 47.5 g * 0.900 J/g C * 37 C
q = 1595.5 J
We need to convert this to calories:
1 cal = 4.184 J
Therefore,
q = 1595.5 J / 4.184 J/cal
q = 381.1 cal
Therefore, the number of calories required for 47.5g of Al to go from 25 to 62 is 381.1 calories.
Learn more about Calories
brainly.com/question/1178789
#SPJ1
The following picture shows the lewis dot structure for Chlorine:
Which of the following is a true statement:
A) Chlorine has a total of 7 electrons
B) Chlorine will lose 1 electron to form a + 1 cation.
C) Chlorine has 1 valence electron
D)Chlorine will gain 1 electron to form a -1 anion

Answers
Lewis dot structure of an atom or molecule represents the valence electrons of the atom. Chlorine have 7 valence electrons and it gains one electron to form an anion with -1 charge. Thus option D is correct.
What is chlorine?Chlorine is 17th element in periodic table. It is 17th group element namely halogen group and have other group members bromine, fluorine, iodine and astatine.
Halogens are highly electronegative with 7 valence electrons. According to octet rule elements with completely filled valence orbitals are stable. Thus halogens like chlorine need one more electron to complete their octet and to be stable.
When atoms gain an electron they acquire a positive charge. Therefore, when chlorine gets one electron through bonding with other atoms it becomes chlorine anion Cl-.
The Lewis dot structure of chlorine represents its 7 valence electrons where 6 electrons are paired and one remains single and this electron on bonding pairs with one electron from another atom and gets negative charge. Hence, option D is correct.
To find more about Lewis dot structure, refer the link below:
https://brainly.com/question/4144781
#SPJ1
Which of the following is a definition of a line segment?
Answers
Answer: So, a line segment is a piece or part of a line having two endpoints. Unlike a line, a line segment has a definite length.
Explanation:
Answer:
a line segment is a part of a line that is bounded by two distinct end points, and contains every point on the line between its endpoints.
Karri spends time finding positive, interesting activities for her 15-year-old daughter, Brooke. Karri encourages Brooke to offer input and choose among the activities. Karri's parental role is best described as
Answers
Karri's parental role can be best described as supportive and collaborative. By spending time finding positive and interesting activities for her daughter Brooke, Karri is showing that she cares about her daughter's well-being and wants her to have a fulfilling life.
Moreover, by encouraging Brooke to offer input and choose among the activities, Karri is demonstrating a collaborative parenting style that fosters independence and decision-making skills in her daughter. This approach is consistent with authoritative parenting, which is characterized by warmth, support, and involvement in a child's life, along with clear expectations and boundaries.
By being involved in her daughter's life and helping her navigate challenges, Karri is promoting positive development and growth in Brooke. Additionally, by involving Brooke in the decision-making process, Karri is teaching her daughter how to make decisions and take responsibility for her choices. Overall, Karri's approach to parenting is one that fosters a positive and collaborative relationship with her daughter, promotes independence and decision-making skills, and helps her daughter navigate the challenges of adolescence.
Learn more about adolescence here-
https://brainly.com/question/9506316
#SPJ11
The crystal structure of NaBr is represented in the diagram above. Which statement correctly compares crystalline NaBr(s) to molten NaBr(l) in terms of electrical conductivity?
Answers
There are no free electrons or other charged particles that can migrate to carry an electrical current in crystalline NaBr. Sodium bromide, however, possesses freely moving ions that can conduct an electric current when it is molten.
An inorganic substance with the formula NaBr is sodium bromide. It is a white, crystalline substance with a high melting point that resembles sodium chloride. It has numerous uses and is a common source of the bromide ion. Crystalline NaBr lacks the electrons necessary to conduct electricity. Still, molten NaBr is formed of freely flowing Na positive ions and Br negative ions, allowing it to be an excellent conductor of electricity.
To know more about ions please click on the below link:
https://brainly.com/question/13692734
#SPJ4
1. Describe what happens to water particles as you increase/decrease the temperature/pressure? (hint: movement/speed, attraction, density, volume, state of matter)
2. Describe and explain what happens to balloons when they are heated/cooled
3. Describe what happens to a cold beverage container on a hot day?
4. Explain how a glass (mercury) thermometer works.
Answers
1) The speed of the particles would increase when heated
2) The balloon would expand when heated and contract when cooled.
3) On a hot day, the pressure of the gas in a beverage increases
4) The mercury thermometer works by expanding or contracting in response to temperature change.
What is effect of temperature?We know that one of the effects of temperature is that it is able change the molecular motion of an object. Thus the molecules of an object are able to move faster when heat is applied and they are able to slow down when the heat is removed.
This is why a balloon would have a greater volume when the temperature is increased as the gas molecules spread out. The volume would reduce or decrease when the temperature is reduced.
Also, on a hot day, a beverage would tend to be more fuzzy as the pressure of the gas in the beverage would increase as the temperature is increased.
Lastly, when we use the mercury thermometer, the volume of the mercury would increase and this is the reason for the expansion of the mercury during temperature measurement.
Learn more about temperature:https://brainly.com/question/11464844
#SPJ1
commercial vinegar was titrated with naoh solution to determine the content of acetic acid, hc2h3o2. a 10.0 ml sample of vinegar was titrated to the endpoint with 20.0 ml of 0.40 m naoh. what was the concentration of acetic acid in the vinegar assuming no other acids were present?
Answers
The concentration of acetic acid in vinegar assuming no other acids were present was 0.8M.
Titration is the laboratory process of quantitative chemical analysis to determine the concentration of an identified analyte.
The reaction is -
HC₂H₃O₂ + NaOH → H₂O + NaC₂H₃O₂
The acetic acid concentration in vinegar was calculated using the following expression.
Ca × Va = Cb × Vb
Ca = Cb × Vb / Va
Ca = 0.40 M × 20 mL / 10.0 mL
= 0.8 M
where,
Ca is the acid concentrationVa is the volume of the acid. Cb is the base concentrationVb is the volume of the base.So,
The concentration of acetic acid in vinegar assuming no other acids were present was 0.8M.
Read more about Titration:
https://brainly.com/question/13031875
#SPJ4
In the following molecules, the primary intermolecular attractive force is

Answers
Answer:
Dipole-dipole
Explanation:
An irregularly-shaped sample of aluminum (Al) is put on a balance and found to have a mass of 25.7 g. The student decides to use the water-displacement method to find the volume. The initial volume reading is 35.5 mL and, after the Al sample is added, the water level has risen to 41.7 mL. Find the density of the Al sample in g/mL. *
Answers
Answer:
4.14516129 g/mL
Explanation:
The density can be found with the following formula.
\(d= \frac{m}{v}\)
where \(m\) is the mass and \(v\) is the volume.
We know the mass is 25.7 grams. We must find the volume.
The volume is equal to the volume of water that is displaced. Subtract the initial volume from the final volume.
⇒ final volume - initial volume
The initial volume is 35.5 mL and the final volume is 41.7 mL.
⇒ 41.7 mL - 35.5 mL
⇒ 6.2 mL
The volume of the aluminum piece is 6.2 mL.
Now we know the mass and the volume.
\(m= 25.7 g\\v=6.2 mL\)
Substitute the values into the formula.
\(d=\frac{m}{v}\)
\(d=\frac{25.7 g}{6.2 mL}\)
\(d=4.14516129 g/mL\)
The density of the aluminum sample is 4.14516129 g/mL
What are the difference between malachite and pyrite and how they are alike

Answers
We can confirm that malachite and pyrite are similar in that they are both carbonate minerals, they differ however in chemical composition and characteristics.
What is malachite and pyrite?Pyrite is a form of iron sulfide. This mineral has come to be known as the fool's gold, for its striking resemblance to gold. Malachite, on the other hand, has been known to closely resemble emeralds and is often used as a gemstone. They are both carbonate minerals, but malachite is copper-based, giving it a different chemical composition and properties.
Therefore, we can confirm that malachite and pyrite are similar in that they are both carbonate minerals, they differ however in chemical composition and characteristics.
To learn more about minerals visit:
https://brainly.com/question/18078524?referrer=searchResults
select the number of moles of co2 formed by the reaction of 0.153 mol c3h8 with excess (non-limiting) o2.
Answers
The number of moles of \(CO_2\) formed by the reaction of 0.153 mol \(C_3H_8\) with excess (non-limiting) \(O_2\) is 0.4593 moles.
What is reaction?Reaction is the process of responding to an event, stimulus, or action. It can be physical, mental, or emotional. Physical reactions can include changes in body temperature, heart rate, respiration, or blood pressure. Mental reactions involve processes such as thought, memory, or perception. Emotional reactions involve expressions of feeling, such as joy, anger, fear, or love. In addition to these physical, mental, and emotional responses, reactions can also be behavioral, or involve taking action.
The number of moles of \(CO_2\) formed by the reaction of 0.153 mol \(C_3H_8\) with excess (non-limiting) \(O_2\) is 0.4593 moles.
This is because the reaction of \(C_3H_8\) and \(O_2\) forms 3 moles of \(CO_2\) for every 1 mole of \(C_3H_8\) that is reacted. Therefore, 0.153 moles of \(C_3H_8\) will produce 0.4593 moles of \(CO_2\) (0.153 x 3 = 0.4593).
To learn more about reaction
https://brainly.com/question/25769000
#SPJ4
A sealed flask contains
3.6 atm H2 gas and
1.8 atm 02 gas.
What is the total pressure in the
flask in atm?
Answers
The total pressure in the flask in atm would be 6.4 atm
Two tennis balls are dropped at the same time. One tennis ball is dropped from the roof of a two-story house. The other tennis ball is dropped from 1 m off the ground. Which tennis ball will be moving the slowest as it hits the ground? Why?