Answers
Answer: 10=14 1=t6
Explanation:
Related Questions
Different "substances" are forms of matter (having volume and taking up space) with different _______________ from each other. a properties b uses c positions d sources
Answers
Answer:
properties
Explanation:
The properties of matter are the various ways in which matter behaves under certain circumstances.
Various substances have different volumes and shapes. These differences account for the various properties of matter.
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation how many moles of N2 can be made when 170.5 grams of CuO are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
Answer:140 grams of N2 are made.
Explanation:
15 mol CuO x (1 mol N2 / 3 mol CuO) = 5 moles of N2.
5 mol N2 x (28 g N2 / 1 mol N2) = 140 grams of N2.
Energy must be removed from a liquid to change it to a solid true or false please hurry it’s a emergency
Answers
false removing energy from a liquid turns it into a gas
Answer:
False
Explanation:
You said fast so I'm just giving the answer
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with
atomic masses of about 1 amu, 2 amu, and 3 amu, respectively. Considering the
average atomic mass of hydrogen that appears on the Periodic Table, which
statement is correct?
Answers
Answer:
sorry i can't figure out the exact answer but i think it is 2amu if it is wrong again i'm so sorry
Explanation:
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu. Then the average atomic mass will be 2 amu. Thus option b is correct.
What are isotopes?Isotopes are defined as a variation of an element that posses same atomic number but different atomic mass.
Isotopes has nearly same chemical behavior by different physical properties.
It can also be defined as the variant of chemical elements that posses same number of protons and electrons but different number of neutrons.
Radioactive isotopes are used in agriculture, food processing, pest control, archaeology and medicines.
Thus, Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu. Then the average atomic mass will be 2 amu. Thus option b is correct.
To learn more about isotopes, refer to the link below:
https://brainly.com/question/11680817
#SPJ5
Your question is incomplete but probably your fell question was
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu, respectively. Considering the average atomic mass of hydrogen that appears on the Periodic Table, which statement is correct?
a. 1.5 amu
b. 2 amu
c. 3 amu
d. 1.01 amu
Calculate the molar mass of the following compound: Ca3P2
Answers
Answer:
The molar mass of the compound given is 182.182 g/mol.
Explanation:
To calculate the molar mass of the compound, we must multiply the number of moles of each element by the the individual molar mass of each element and add them together.
Let's start with Calcium. The molar mass of Calcium is 40.078. In this compound, we have three moles of Calcium, so we should multiply this number by 3.
40.078 g/mol * 3 mol = 120.234 g
Now, let's do the same for Phosphorus.
30.974 g/mol * 2 g/mol = 61.948 g
To find the molar mass of the entire compound, we should add these two values together.
120.234 g + 61.948 g = 182.182 g
Therefore, the correct answer is 182.182 g/mol.
Hope this helps!
Explain in a three-paragraph essay the mechanics of how a battery works. How does the choice of metals used in a battery affect its performance? what specific metals work best?
Answers
A battery is a device that converts chemical energy into electrical energy through a process known as an electrochemical reaction.
How does a battery work ?When a battery is connected to a circuit, the electrochemical reaction causes a flow of electrons from the anode to the cathode, generating an electric current that can power a device.
The metal chosen for the anode must be capable of losing electrons easily, while the metal chosen for the cathode must be capable of accepting electrons. The choice of metals can also affect the voltage and capacity of the battery, as well as its overall efficiency.
In general, the metals used in a battery should have a large difference in their electronegativity values, which determines how easily an atom can attract electrons. Common metals used in batteries include zinc, lithium, nickel, and cadmium.
Find out more on batteries at https://brainly.com/question/16553902
#SPJ1
What is the number of atoms of oxygen in 0.1 mol of CuSO4.5H2O? & explain
Answers
Again, 1 mole CuSO4.5H2O contains 9 oxygen atoms
Therefore,1 mole CuSO4.5H2O contains 9 × 6.023×10^23 no.of oxygen atoms
Hope this help!
tiana is a chemist who is making a chemical to add to swimming pools
Answers
Tiana is developing a chemical additive to be used in swimming pools.
What is chemical additive?Chemical additives are substances added to products to alter or improve their performance. They are used in a wide range of consumer products and industrial processes for a variety of purposes including improving shelf-life, enhancing flavor, or increasing the efficiency of a process. Common examples of chemical additives are preservatives, colorants, emulsifiers, antioxidants, stabilizers, and thickeners.
This additive is designed to help keep the pool clean and sanitary, by removing bacteria and other contaminants from the water. The additive is also designed to help balance the pH of the pool water, to ensure that it is safe for swimming and does not irritate swimmers' skin or eyes. The chemical additive must also be safe to use, and must not cause any adverse reactions in swimmers. Tiana's work involves testing different chemical compounds to find the most effective and safe additive for pool water.
To learn more about bacteria
https://brainly.com/question/30307005
#SPJ1
PLEASE HELP ASAP!!!
ANSWER FOR PHOTO 1
STUDENT 1
STUDENT 2
STUDENT 3
STUDENT 4
ANSWERS FOR PHOTO 3
A
B
C
D
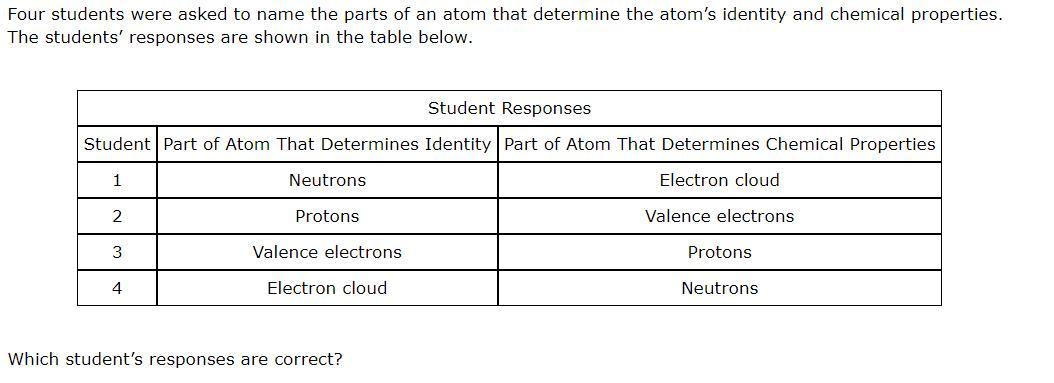




Answers
The student that gave the correct response is student 3.
What is the correct response?We know that there are three main subatomic particles that we can be able to find in the atom and these are;
ProtonsElectronsNeutronsWe know that the electrons can be classified in to groups based on the valence electrons that the elements have and this is what we can be able to use to judge the reactivity of the atoms of the elements as we know in chemistry.
Hence, the valence electrons determine the chemical reactivity of the atom.
Learn more about valence electrons:https://brainly.com/question/28977387
#SPJ1
Question 2 A soil with pH 8.0, EC 8.0, and ESP 25% would be: a. sodic saline b. saline-sodic c. none of these d. all of these
Answers
A soil with pH 8.0, EC 8.0, and ESP 25% would be saline-sodic.
The soil would be classified as "sodic saline" because it has a high ESP value (25%) which indicates a high sodium content, and a high pH value (8.0) which indicates alkalinity. The EC value (8.0) indicates high salinity, but this alone does not necessarily make the soil saline-sodic.
A soil is considered sodic if its ESP (Exchangeable Sodium Percentage) is greater than or equal to 15%. Since the given ESP is 25%, the soil is sodic. Additionally, a soil is considered saline if its EC (Electrical Conductivity) is greater than or equal to 4 dS/m. In this case, the EC is 8.0, making the soil saline.
To know more about pH,
https://brainly.com/question/15289741#
#SPJ11
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
net equation of fatty acid synthesis write the net equation for the biosynthesis of palmitate in rat liver, starting from mitochondrial acetyl-coa and cytosolic nadph, atp, and co2.
Answers
The net equation for the synthesis for the biosynthesis of palmitate(16-carbon fatty acid) in rat liver, starting from mitochondrial acetyl-CoA and cytosolic NADPH, ATP, and CO2:
8 Acetyl CoA (2C) + 14 NADPH + 13H+ + 7 ATP→ Palmitate (16C) + 8 CoA-SH + 6 H2O + 14 NADP+ + 7 ADP + 7 Pi
In this equation, 8 acetyl-CoA molecules are used, along with 14 NADPH, 7 ATP, to produce 1 molecule of palmitate. Additionally, 14 NADP+, 8 CoA, 6 H2O, 7 ADP, and 7 Pi molecules are generated as byproducts during the fatty acid synthesis process.
Learn more about palmitate : https://brainly.com/question/15711728
#SPJ11
Given the electrochemical reaction, , what is the value of Ecell at 25 °C if [Mg2+] = 0.100 M and [Cu2+] = 1.75 M?
Half-reaction
E° (V)
+1.40
+1.18
+0.80
+0.54
+0.34
-0.04
-1.66
-2.37
-2.93
+2.75 V, +2.67 V, +2.79 V, -2.00 V, +2.71 V
15.
Which statement about pure water is correct? Pure water does not ionize, pH > pOH, pH = 7 for pure water at any temperature, Kw is always equal to 1.0 × 10-14, OR [H3O+] = [OH-]?
17. The standard cell potential for the reaction is 1.104 V. What is the value of Ecell at 25 °C if [Cu2+] = 0.250 M and [Zn2+] = 1.29 M?
+1.083 V
–1.104 V
+1.104 V
+1.062 V
+1.125 V
Answers
1. The value of Ecell at 25 °C for the given electrochemical reaction, where [Mg²⁺] = 0.100 M and [Cu²⁺] = 1.75 M, is approximately +2.75 V.
15. The value of Ecell at 25 °C for the given electrochemical reaction, where [Mg²⁺] = 0.100 M and [Cu²⁺] = 1.75 M, is approximately +2.75 V.
17. The value of Ecell at 25 °C for the given standard cell potential of 1.104 V, with [Cu²⁺] = 0.250 M and [Zn²⁺] = 1.29 M, is approximately +1.083 V.
1. To calculate the cell potential (Ecell) at 25 °C, we need to use the Nernst equation:
Ecell = E°cell - (RT/nF) * ln(Q)
Given the concentrations of [Mg²⁺] and [Cu²⁺] in the reaction, we can determine the reaction quotient (Q). Since the reaction is not specified, I assume the reduction half-reaction for copper (Cu²⁺ + 2e⁻ → Cu) and the oxidation half-reaction for magnesium (Mg → Mg²⁺ + 2e⁻).
Using the Nernst equation and the given E° values for the half-reactions, we can calculate the value of Ecell:
Ecell = E°cell - (0.0257 V/K * 298 K / 2) * ln([Cu²⁺]/[Mg²⁺])
= 2.75 V - (0.0129 V) * ln(1.75/0.100)
≈ 2.75 V - (0.0129 V) * ln(17.5)
≈ 2.75 V - (0.0129 V) * 2.862
≈ 2.75 V - 0.037 V
≈ 2.713 V
Therefore, the value of Ecell at 25 °C for the given reaction with [Mg²⁺] = 0.100 M and [Cu²⁺] = 1.75 M is approximately +2.75 V.
15. Kw, the ion product of water, represents the equilibrium constant for the autoionization of water: H₂O ⇌ H₃O⁺ + OH⁻. In pure water, at any temperature, the concentration of both H₃O⁺ and OH⁻ ions is equal, and their product (Kw) remains constant.
Kw = [H₃O⁺][OH⁻] = 1.0 × 10⁻¹⁴
This constant value of Kw implies that the product of [H₃O⁺] and [OH-] in pure water is always equal to 1.0 × 10⁻¹⁴ at equilibrium. The pH and pOH of pure water are both equal to 7 (neutral), as the concentration of H₃O⁺ and OH⁻ ions are equal and each is 1.0 × 10⁻⁷ M.
Therefore, the correct statement about pure water is that Kw is always equal to 1.0 × 10⁻¹⁴.
17. Given the reduction half-reaction for copper (Cu²⁺ + 2e⁻ → Cu) and the oxidation half-reaction for zinc (Zn → Zn²⁺ + 2e⁻), the overall reaction can be written as:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Using the Nernst equation and the given E°cell value, we can calculate the value of Ecell:
Ecell = E°cell - (0.0257 V/K * 298 K / 2) * ln([Zn²⁺]/[Cu²⁺])
= 1.104 V - (0.0129 V) * ln(1.29/0.250)
≈ 1.104 V - (0.0129 V) * ln(5.16)
≈ 1.104 V - (0.0129 V) * 1.644
≈ 1.104 V - 0.0212 V
≈ 1.083 V
Therefore, the value of Ecell at 25 °C for the given standard cell potential of 1.104 V, with [Cu²⁺] = 0.250 M and [Zn²⁺] = 1.29 M, is approximately +1.083 V.
To learn more about electrochemical reaction, here
https://brainly.com/question/31236808
#SPJ4
which is the correct name for the compound n2o4? a. nitrogen oxide b. tetranitrogen dioxide c. dinitrogen quadoxide d. dinitrogen tetroxide
Answers
The correct name for the compound N₂O₄ is dinitrogen tetroxide. The formula N₂O₄ represents the molecule dinitrogen tetroxide.
Hence, option D (dinitrogen tetroxide) is the correct answer.
The formula for nitrogen monoxide is NO, nitrogen dioxide is NO₂, and nitrogen trioxide is N₂O₃. The term "tetranitrogen dioxide" (Option B) is incorrect as it contains the prefix "tetra-," which refers to four. There are only two nitrogen atoms present in the molecule.
The term "dinitrogen quadoxide" (Option C) is also incorrect because it does not exist. The term quadoxide does not apply to any known compound.
Hence, the correct name of the compound N₂O₄ is dinitrogen tetroxide (Option D).
Learn more about molecules at:
https://brainly.com/question/30623065
#SPJ11
pls answer question will mark brainliset tyty

Answers
The option (1) water supply i.e. the biotic factor that affect the human most.
Competition is a biotic element that prevents population increase in an aquatic habitat since it involves living things.Competition is an interaction between individuals or species where the presence of another reduces the fitness of one. A factor may be the limited availability of at least one resource (such as food, water, and territory) used by both.Abiotic elements including as depth, sunlight, nutrients, and oxygen availability have an impact on population increase in aquatic ecosystems.An ecosystem's overall health is also influenced by biotic factors such as the diversity of consumers and the presence of autotrophs, or self-sustaining organisms like plants. Abiotic variables have an impact on an organism's capacity for survival and reproduction. Abiotic barriers prevent the formation of population .
To know more about biotic factor
https://brainly.com/question/27430655
#SPJ1
4. Two liquids at the same temperature are mixed together. You
observe no change in color and no gas or solid formed. Which of
the following would you check to see if a chemical change may have occurred ?
Answers
Answer:
I would say you would check for temperature change. A chemical reaction is shown by a change in color, a change in temperature, solids forming, odor, or smoke. So check temperature.
edit: while odor is an option in the answer. i think temp is a better choice.
Answer:
Check temp change
Explanation:
Never taste
HELP PLEASE !!!
Name the Salt formed When Nitric acid + Magnesium hydroxide
Answers
Answer:
magnesium chloride
Explanation:
Complete step-by-step answer:
The remaining ions combine to produce the salt of neutralisation. The salt is magnesium chloride. The chemical formula for the compound is $MgC{{l}_{2}}$.
8
Type the letter that represents the correct location
for each particie type below.
The neutron is found at
The electron is found at
The proton is found at
Answers
The neutron is found in the nucleus
The electron is found outside the nucleus
The proton is found in the nucleus
What is an element? Arrow

Answers
Answer:
One arrow is positioned in each box according to Hund's Rule which tells us to maximise the number of unpaired electrons in orbitals of the same subshell, and, to give those electrons the same "spin" (parallel spin).
Explanation:
A student is doing an investigation on the movement of objects.
Why is it important for her to repeat the investigation several times?
Answers
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.
Answers
Percent yield = 70%
Further eplanationPercent yield is the comparison of the amount of product obtained from a reaction with the amount you calculated
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
An actual yield is the amount of product actually produced by the reaction. A theoretical yield is the amount of product that you calculate from the reaction equation according to the product and reactant coefficients
Reaction
2H₂ (g) + O₂ (g) → 2H₂O (g)
mass of H₂O (theoretical) :\(\tt mass=mol\times MW(mol~ratio~H_2O\div H_2=2\div 2)\\\\mass=(\dfrac{2}{2}\times \dfrac{13.8}{2})\times 18~g/mol\\\\mass=124.2~g\)
percent yield\(\tt \%yield=\dfrac{87}{124.2}\times 100\%=\boxed{\bold{70\%}}\)
Many ski resorts and mountain cities can be reached directly by planes which deposit travelers a mile or more above sea level. This can result in altitude sickness due to hypoxemia, or reduced oxygen in the blood, caused by the unaccustomed exposure to the lower atmospheric pressure at high elevations. On a given day, the prevailing atmospheric pressure in Albuquerque, NM (elevation 1620 meters) might be 0.799 atm. Calculate this pressure expressed in mmHg and in torr. Relationship between altitude and barometric pressure.
Answers
Answer:
See explanation
Explanation:
To convert from atm to mmHg
1 atm = 760 mmHg
0.799 atm = 0.799 atm * 760 mmHg/1 atm = 607.24 mmHg
To convert from atm to torr
1 atm = 760 torr
0.799 atm = 0.799 atm * 760 torr/1 atm = 607.24 torr
What is 1 mole of CO2?
Answers
1 mole of CO2 is 6.022 x 10^23 molecules of CO2. It is also 44.01 g of CO2. Here 44.01 g comes as the sum of individual atoms of CO2 i.e. 12.01 + 16 + 16 i.e. 44.01 g
CO2 is composed of one carbon atom and two oxygen atoms, which have the atomic masses of 12.01 g/mol and 16.00 g/mol respectively. So, the molar mass of CO2 is the sum of the atomic masses of the atoms that make up CO2, which is 12.01 + 16.00 + 16.00 = 44.01 g/mol. Carbon dioxide (CO2) is a naturally occurring compound made up of one carbon atom and two oxygen atoms. It is a colorless, odorless gas and is a byproduct of many biological and industrial processes. CO2 is a greenhouse gas, which means it helps trap heat in the Earth's atmosphere and contributes to global warming.
Therefore, one mole of CO2 weighs 44.01 grams.
To know more about mole please refer: https://brainly.com/question/20486415
#SPJ4
1. Explain the relationship
between Polaris and Earth's tilt
Answers
Answer:
that it i think
Explanation:
the earth revolves around the Sun once each year and spins on its axis of rotation once each day. This axis of rotation is tilted 23.5 degrees relative to its plane of orbit around the Sun. The axis of rotation is pointed toward Polaris the North Star. As the Earth orbits the Sun the tilt of Earth’s axis stays lined up with the North Star.
In which group do all the elements have the same number of valence electrons?
a. p, s, cl
b. ag, cd, ar
c. na, ca, ba
d. p, as, se
e. none of these
Answers
The correct option is (e) none of these ,Valency is the ability of an element to combine.
What is Valency?Valency is the ability of an element to combine. Elements in the same group of the periodic table have the same valency. An element's valency is determined by how many electrons make up its outer shell.
A property of an element called "valency" defines how effectively it can meld with other elements. It is a way to count how many electrons are in an atom's outermost shell.
An atom's stability is determined by its valence electrons. Additionally, valence electrons are in charge of an atom's stability. The Octet Rule states that atoms often gain or lose electrons to reach the octet state, which is defined as having eight electrons in their valence shell. They stabilise at this stage.
To learn more about valency go to -
https://brainly.com/question/13552988
#SPJ4
Calculate the effective nuclear charge on a valence electron in a bromine atom.
Answers
The effective nuclear charge (Zeff) is the net positive charge experienced by an electron in an atom, taking into account the shielding effect of inner electrons.
Bromine (Br) has 35 electrons. The atomic number of bromine is 35, which means it has 35 protons in its nucleus.
The valence shell of bromine is the fourth energy level, which contains 7 electrons. To calculate the effective nuclear charge on a valence electron in a bromine atom, we need to consider the following:
The atomic number of the element (Z) = 35
The number of inner electrons (n) = 28
The number of valence electrons (s) = 7
Using Slater's rules for calculating Zeff, we can obtain the effective nuclear charge on a valence electron in a bromine atom:
Zeff = Z - (0.35 x n) - (0.85 x s)
Zeff = 35 - (0.35 x 28) - (0.85 x 7)
Zeff = 35 - 9.8 - 5.95
Zeff = 19.25
Therefore, the effective nuclear charge on a valence electron in a bromine atom is approximately 19.25.
Want to know more about nuclear charges visit the link which is given below;
https://brainly.com/question/13664060
#SPJ4
Each student will write up their own lab report and turn it in
Answers
Here are some general steps you can follow to write a lab report:
The StepsUnderstand the purpose of the lab report: Before you begin writing, make sure you understand the purpose of the lab report. What are the objectives of the experiment? What are the research questions being investigated? What hypothesis is being tested?
Gather your data: Make sure you have all the data you need to write your report. This includes raw data, observations, and any notes you took during the experiment. Organize your data in a clear and logical manner so that you can easily refer to it when writing your report.
Write an outline: Create an outline for your report that includes the main sections you need to cover. These typically include an introduction, methods, results, discussion, and conclusion.
Write the introduction: The introduction should provide an overview of the experiment and explain its significance. You should also provide some background information to help the reader understand the context of the experiment.
Write the methods: In the methods section, describe the experimental design, materials used, and procedures followed. Be sure to include enough detail so that someone else could repeat the experiment.
Write the results: In the results section, present your data in a clear and organized manner. Use tables, graphs, and figures to help illustrate your findings. Make sure to include any statistical analyses you performed.
Write the discussion: In the discussion section, interpret your results and explain what they mean. Discuss any patterns or trends you observed and explain how they relate to the research question. Compare your results to previous research in the field, and discuss any limitations or potential sources of error.
Write the conclusion: The conclusion should summarize the main findings of the experiment and explain their significance. You should also discuss any future directions for research in the field.
Proofread and revise: Once you have completed your first draft, proofread your report carefully to check for errors and inconsistencies. Revise your report as necessary to make sure it is clear, concise, and well-organized.
Read more about lab report here:
https://brainly.com/question/29500102
#SPJ1
Carbonic acid, H2CO3
, can be found in a wide variety of body fluids (from dissolved CO2
). a Calculate the hydronium-ion concent…
Carbonic acid, H2CO3
, can be found in a wide variety of body fluids (from dissolved CO2
). a Calculate the hydronium-ion concentration of a 6.00×10−4MH2CO3
solution. What is the concentration of CO32−?
Answers
The concentration of CO₃²⁻ in the hydronium-ion concentration of a 6.00×10−4M H₂CO₃ solution will be 4.3 × 10^-11 M.
The dissociation of carbonic acid can be represented by the following equation:
H₂CO₃(aq) + H₂O(l) ⇌ HCO₃−(aq) + H₃O+(aq)
The equilibrium constant for this reaction, Ka, is 4.3 × 10^-7.
We can use the equilibrium constant to calculate the concentration of hydronium ions in a solution of carbonic acid.
[H₃O+] = [HCO₃−]Ka/[H₂CO₃]
[H₃O+] = (6.00 × 10^-4) × 4.3 × 10^-7 / 6.00 × 10^-4
[H₃O+] = 4.3 × 10^-11 M
The concentration of carbonate ions can be calculated using the following equation:
[CO₃²⁻] = [H₂CO₃]Ka/[H₃O+]
[CO₃²] = (6.00 × 10^-4) × 4.3 × 10^-7 / 4.3 × 10^-11
[CO₃²⁻] = 6.00 × 10^-4 M
Therefore, the hydronium ion concentration of a 6.00×10−4M H₂CO₃ solution is 4.3 × 10^-11 M, and the concentration of carbonate ions is 6.00 × 10^-4 M.
To know more about concentration of hydronium-ion, refer here:
https://brainly.com/question/28173930#
#SPJ11
1. 2 NH3 + 3 CuO g 3 Cu + N2 + 3 H2O In the above equation how many moles of water can be made when 36 moles of NH3 are consumed?
2. 3 Cu + 8HNO3 g 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of NO can be made when 86 moles of HNO3 are consumed?
3. 3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 82 moles of HNO3 are consumed?
Sodium chlorate decomposes into sodium chloride and oxygen gas as seen in the equation below.
4. 2NaClO3 --> 2NaCl +3O2
How many moles of NaClO3 were needed to produce 56 moles of O2? Round your answer to the nearest whole number.
Answers
First we find the number of moles for HN3 which is 1 before multiplying it times the number before the element which is 2.
We then find the moles for H20 which is 1 before multiplying it times the number before the element which is 3. We now have 3 numbers. 36 moles NH3, 2 moles NH3, and 3 moles H20. We then cross multiple the moles of NH3 which is 36 with the moles for NH3 which is 3 making it 108 before dividing it by two which gives us 54 moles H20 as our answer.
2. 86 moles HNO3 - ? moles NO
We first find the moles for HNO3 which is 1 before multiplying it with the number in front of it which is 8. We soon find the miles for NO which is 1 before multiplying it with 2. We then cross multiply We soon multiply 86 with 2 which leads to 172 before divide by 8 which leads us with 21.5 moles NO.
3. 82 moles HNO3 - ? moles H2O
We find the moles for HNO3 which is 1 and multiply it by 8. We then get the moles for H20 which is 1 before multiplying it by 4. When then cross multiply 82 with 4 which is 328 before dividing it by 8 which leaves us with 41 moles H2O.
4. 56 moles O2 - ? moles NaClO3
We find the moles for O2 which is 1 before multiplying it with 3. We then find the moles for NaClO3 which is 1 before multiplying it with 2. We The cross multiply 56 with 2 to get 112 before dividing it by 3 which gives us 37.33 which rounds to 37 miles NaClO3.
beers law lab if your unknown copper sulfate solution was produced by diluting 20 ml of a more concentrated copper sulfate solution to a final volume of 100 ml, what was the original concentration of the concentrated solution?
Answers
100.0 mL of a solution containing copper was mixed in it. At 620 nm, this solution's absorbance was 0.477.
What does the focused meaning mean?Contained, present, or taking place in a constrained region or area: not dispersed. a light beam that is extremely focused. 3. fervent, fervent a task requiring numerous hours of focused work.
What does liquid concentrated mean?A liquid that has had its water removed has been strengthened by concentration. Use concentrated apple or apricot juice, honey, or both moderately to sweeten food. Condensed, rich, undiluted, and reduced are synonyms for Additional words for concentration.
To know more about Concentrated visit:
https://brainly.com/question/10725862
#SPJ4