Answers
Answer:
2. 232 & 86
Explanation:
Related Questions
What are you measuring when
you step on a scale?
A. the amount of Earth's gravity pulling you
down
B. the amount of atoms you contain
C. how high you can jump
D. your volume
Answers
Answer:
B. the amount of atoms you contain
the law of definite proportions states that substances combine in predictable proportions and that excess reactants remain unchanged. true false
Answers
Using the concepts of Avogadro Law, we got the given statement is true.
The law of definite proportions defines samples of a compound will always contain the same proportion of the elements by mass. The mass ratio of elements is fixed no matter where the elements comes from, how the compound is prepared. Essentially, the law is based on fact that an atom of a particular element is the same as any other atom of that element. So, an atom of oxygen is same, whether it comes from silica or oxygen in the air.
The Law of Constant Composition is equivalent to definite proportions, which states each sample of a compound has the same composition of elements by mass.
The law of definite proportions actually tells water will always contain 1/9 hydrogen and 8/9 oxygen by mass.
The sodium and chlorine in table salt combine according to the rule and become NaCl. The atomic weight of sodium is 23grams and that of chlorine is about 35grams, so from the law one may conclude dissociating 58 grams of NaCl will produce about 23 g of sodium and 35 g of chlorine.
Hence, it is true that the substances combine in predictable proportions and that excess reactants remain unchanged.
To know more about the Avogadro Law, visit here:
https://brainly.com/question/4133756
#SPJ4
Ocean currents bring warm from the equator towards earth?
Answers
Answer:Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
Explanation:
Answer:
Explanation:
Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
hope it helps!
Filter the air that we inhale and exhales carbon dioxide
Answers
Air filters are devices designed to remove impurities and particles from the air, improving its quality and making it healthier to breathe. While air filters can help remove certain contaminants, such as dust, pollen, and pet dander, they do not specifically filter out carbon dioxide (CO2).
Carbon dioxide is a natural component of the air we exhale, and its concentration in the atmosphere is regulated through natural processes, such as photosynthesis by plants. The removal of carbon dioxide from the air typically occurs through the natural carbon cycle, where plants absorb CO2 during photosynthesis and release oxygen.
If you are looking to reduce the carbon dioxide levels indoors, the most effective method is to ensure proper ventilation in the space. This can be achieved by opening windows or using mechanical ventilation systems to bring in fresh outdoor air and remove stale air. Additionally, increasing the number of plants indoors can help absorb carbon dioxide and release oxygen through photosynthesis.
It's important to note that while air filters can improve indoor air quality by removing various pollutants, they are not designed to specifically target or remove carbon dioxide.
Learn more about carbon dioxide Here-
https://brainly.com/question/14445045
#SPJ4
What is the mass percent (m/m) of glucose in a solution that contains 30.0 g of water and 5.8 g of glucose?
A) 16.2%
B) 19.3%
C) 14.4%
D) 5.8%
Answers
The mass percent (m/m) of glucose in a solution that contains 30.0 g of water and 5.8 g of glucose is 16.2%.
Thus, the correct option is A.
Mass percent is a term used to describe the concentration of a solution. It is defined as the mass of the solute in grams divided by the total mass of the solution in grams, multiplied by 100 percent.
To find the mass percent of glucose in the given solution, we need to calculate the total mass of the solution. The total mass of the solution is given by the sum of the mass of glucose and water:
30.0 g (water) + 5.8 g (glucose) = 35.8 g (total mass of solution)
The mass percent of glucose in the solution is then calculated as follows:
Mass percent (m/m) = (mass of glucose ÷ total mass of solution) × 100 percent
= (5.8 ÷ 35.8) × 100 percent= 16.2 percent
Therefore, the mass percent (m/m) of glucose in the solution is 16.2%.
For more information about mass percent refers to the link: https://brainly.com/question/9904990
#SPJ11
guys- my science teacher doesn't teach, he just says go on schoology
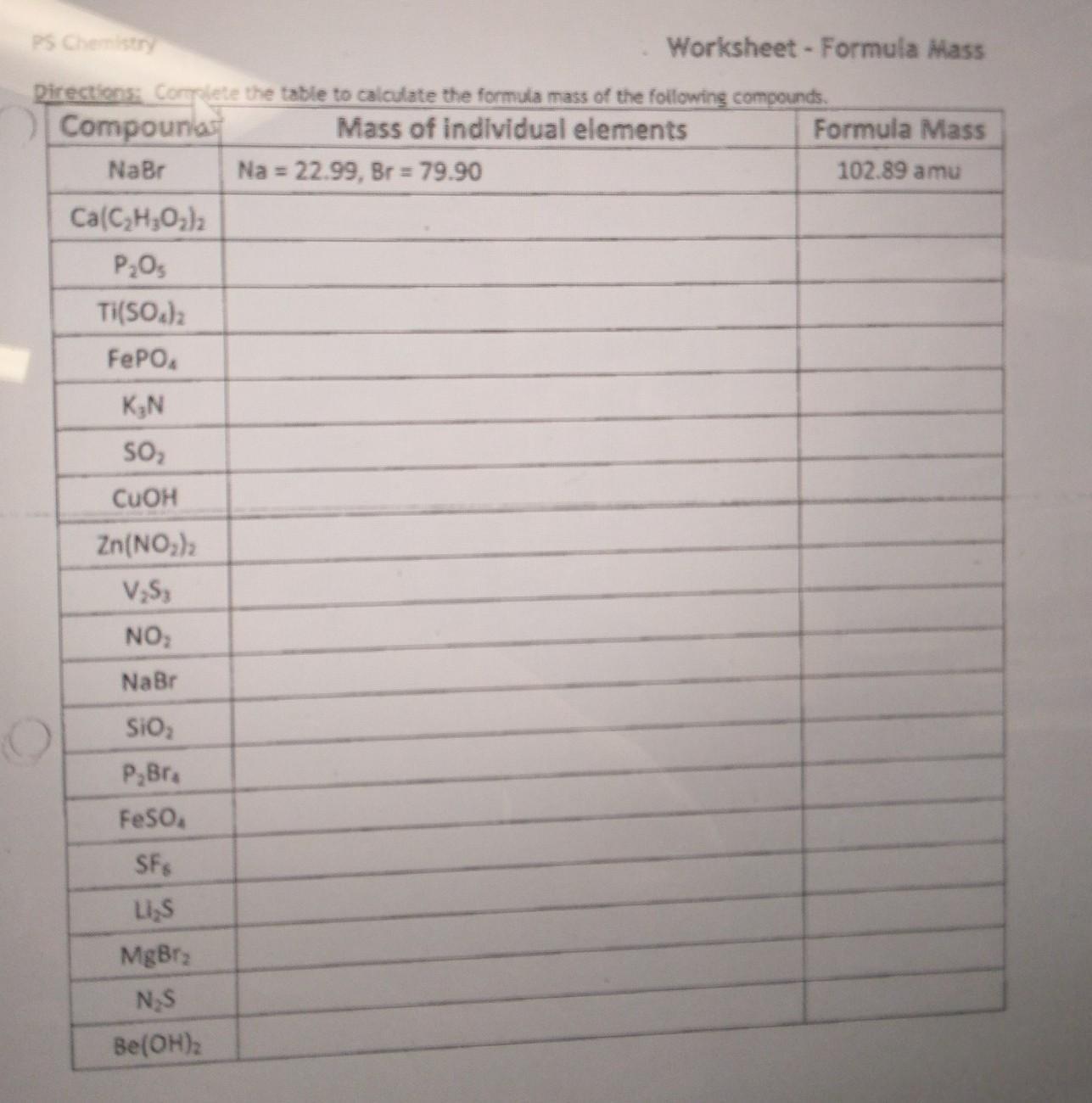
Answers
How many moles are in 8.11 x 10e20 molecules of CH4
Answers
Answer:
0.0013 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{8.11 \times {10}^{20} }{6.02 \times {10}^{23} } \\ = 0.0013471\)
We have the final answer as
0.0013 molesHope this helps you
The pressure P (in kilopascals), volume V (in liters), and temperature T (in kelvins) of a mole of an ideal gas are related by the equation PV = 8.31T . Find the rate at which the volume is changing when the temperature is 290 K and increasing at a rate of 0.15 K/s and the pressure is 14 and increasing at a rate of 0.05 kPa/s.
Please show your answers to at least 4 decimal places.
(dV)/(dt) = Box LI
Answers
The rate at which the volume is changing when the temperature is 290 K and increasing at a rate of 0.15 K/s and the pressure is 14 kPa and increasing at a rate of 0.05 kPa/s is approximately -0.9474 L/s.
The rate at which the volume is changing when the temperature is 290 K and increasing at a rate of 0.15 K/s, and the pressure is 14 kPa and increasing at a rate of 0.05 kPa/s can be found using implicit differentiation.
Given: PV = 8.31T
To find dV/dt, we need to differentiate both sides of the equation with respect to time (t) while considering the chain rule:
P(dV/dt) + V(dP/dt) = 8.31(dT/dt)
Substituting the given values:
14(dV/dt) + 290(0.05) = 8.31(0.15)
Rearranging the equation to solve for dV/dt:
14(dV/dt) = 1.2465 - 14.5
14(dV/dt) = -13.2535
(dV/dt) = -13.2535/14
(dV/dt) ≈ -0.9474
To know more about temperature click here,
https://brainly.com/question/7510619
#SPJ11
Describe how you would prepare a pure dry sample of lead(II) sulfate crystals starting from solutions of lead(II) nitrate and sodium sulfate.
Include a series of key steps in your answer.
Answers
Answer:
Method: Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker. Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod.
what is it called when a plane goes 2 as fast as sound
Answers
(Digestion, Intestine, Bile)is secreted by the liver to help in the breaking down of food particles.
Answers
Answer:
Bile
Explanation:
Bile is secreted by the liver to help in the breaking down of food particles.
A scientist is studying rock formations. She notices some unusual rocks like those pictured here. What can she conclude about this area? It was part of a slow underwater eruption. It was formed at a subduction zone. It is moving toward a mid-ocean ridge. It is a very young piece of land.
Answers
Answer:
It was part of a slow underwater eruption.
Explanation: Or just A (:
Answer:
i agree with the human above me
Explanation:
love ye have a great day but i didnt have a good day bc my uncle ate my cheese puffs :'( anyways i got a 100 on the assaignment and this was the only answer i had to look up im proud of me
A solution contains 1. 60g of trioxonitrate(v) acid in 250cm³ of solution,B contains 10. 0gdm-³ of XHCO3. 25. 00cm³ of 24. 90cm³ A for complete neutralize. Calculate the
1. Concentration of acid in A in moldm-³
2. Concentration of XHCO3in B in moldm-³
3. Molar mass of XHCO3
4. Value of X
Equation reaction,HNO3(aq)+XHCO3 (aq) react XNO3(aq)+CO2(g)+h2o
Answers
Answer:awrf
Explanation:
the chemical reaction for combustion of any hydrocarbon follows a pattern: hydrocarbon oxygen gas yields carbon dioxide water any fossil fuel will produce the same products and release energy when burned in oxygen. how is cellular respiration similar to the general combustion reaction?
Answers
Both cellular respiration and burning (combustion) include the dissolution of chemical bonds, the use of oxygen, the generation of carbon dioxide, and the release of energy. However, there are some key differences between the two processes.
The fundamental chemical process of releasing energy from a fuel and air mixture is combustion, sometimes referred to as burning. In an internal combustion engine (ICE), the gasoline is ignited and burned inside the engine itself. The energy from the combustion is then partially converted into work by the engine.
Cellular respiration is a series of chemical reactions that break down glucose to produce ATP, which may be used as energy to power many reactions throughout the body. There are three main steps of cellular respiration: glycolysis, the citric acid cycle, and oxidative phosphorylation.
T o know more about Cellular Respiration click here:
https://brainly.com/question/29760658
#SPJ4
a phenomenon known as ____ occurs under conditions where a strong selection pressure acts on a particular change in an amino acid
Answers
The phenomenon known as "positive selection" occurs under conditions where a strong selection pressure acts on a particular change in an amino acid.
Positive selection is a process that leads to the fixation of a beneficial mutation in a population, and it is often associated with adaptations to new environmental conditions or host-pathogen interactions.
Positive selection can be detected by comparing the rates of non-synonymous (amino acid-changing) and synonymous (silent) substitutions in protein-coding genes, and it has important implications for understanding the evolution of molecular pathways and the emergence of novel functions.
To know more about host-pathogen interactions:
https://brainly.com/question/28209790
#SPJ11
40 points. Explain how a substituted hydrocarbon is made.
Answers
Answer: Organic acids form when a carboxyl group (−COOH) is substituted for one of the hydrogen atoms in a hydrocarbon.
In the early 1600's, Galileo used a telescope to investigate dark features on the Sun's surface and used them to calculate the rate of the sun's rotation. What areas of relatively cool gas were Galileo observing?
prominences
solar flares
photospheres
sunpsots
Answers
Answer:
I think its "sunspots"
Explanation:
RNA pol III: Which of the following proteins or complexes assists in melting the promoter?
TBP
UBF1
B"
TFIIIC
Brf
Answers
RNA polymerase III (pol III) is a critical enzyme that transcribes small non-coding RNAs, such as tRNAs, 5S rRNA, and other small regulatory RNAs.
For transcription to occur, pol III needs to recognize and bind to the promoter sequence upstream of the coding region.
Several proteins and complexes are involved in this process, including TBP, UBF1, TFIIIC, Brf, and B". Among these, TFIIIC and B" are responsible for unwinding the DNA double helix, which is necessary for transcription initiation.
Specifically, TFIIIC recruits B", which then binds to the promoter and initiates the melting of the DNA. Once the DNA is melted, TBP and Brf can bind to the promoter, allowing pol III to initiate transcription.
Overall, the interplay between these proteins and complexes is essential for efficient and accurate transcription by RNA pol III.
To know more about RNA. please visit.....
brainly.com/question/32289417
#SPJ11
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If a 1.67 g sample of methane is burned at constant pressure, what will be the value of ∆H
Answers
Answer:
\(\Delta H=-92.7kJ\)
Explanation:
Hello there!
In this case, according to the given information, we can infer that 890 kJ of energy are released when 1 mole of methane is burned; however, to find the total heat when 1.67 grams are burned, we first need to calculate the moles in this mass of methane:
\(1.67gCH_4*\frac{1molCH_4}{16.04gCH_4}=0.104molCH_4\)
And thus, for calculating the resulting ∆H, we proceed as follows:
\(\Delta H=-890kJ/mol*0.104mol\\\\\Delta H=-92.7kJ\)
Regards!
which of the following are arrhenius bases? a. h3aso4 b. ba(oh)2 c. hclo d. koh
Answers
The Arrhenius bases among the given options are Ba(OH)2 and KOH. Arrhenius bases are substances that, when dissolved in water, release hydroxide ions (OH-) as the predominant species. Among the given options:
a. H3AsO4: This is not an Arrhenius base. It is a polyprotic acid, known as arsenic acid, and releases protons (H+) when dissolved in water, making it an acid.
b. Ba(OH)2: This is an Arrhenius base. When Ba(OH)2 is dissolved in water, it dissociates into Ba2+ ions and hydroxide ions (OH-). The hydroxide ions make it a base.
c. HClO: This is not an Arrhenius base. It is a weak acid, known as hypochlorous acid, and releases protons (H+) when dissolved in water.
d. KOH: This is an Arrhenius base. When KOH is dissolved in water, it dissociates into K+ ions and hydroxide ions (OH-). The presence of hydroxide ions makes it a base.
Therefore, the Arrhenius bases among the given options are Ba(OH)2 and KOH.
To learn more about Arrhenius bases click here : brainly.com/question/9936252
#SPJ11
A sample of fluorine gas is confined in a 5.0-L container at 0.432 atm and 37 °C. How many moles of gas are in this sample?
Answers
Answer:
About 0.08486 moles
Explanation:
PV=nRT, when P is the pressure in atmospheres, V is the volume in liters, n is the number of moles, R is the universal gas constant, and T is the temperature in Kelvin.
\(0.432 \cdot 5= n \cdot 0.0821 \cdot 310\)
\(n\approx 0.08486\) moles
Hope this helps!
How much heat will be absorbed by 320.0 g of water when its temperature is raised by 35.0°C? The specific heat for water is 4.184 J/g°C.
Answers
Answer:
46860.8 joules of heat
Explanation:
Use the formula ΔQ = mcΔT
ΔQ = gain or loss of heat (in joules)
M = mass (in grams),
C = specific heat (in J/g°C)
ΔT = change in temperature (in °C)
Substitute the known values into the formula:
ΔQ = (320.0 g)(4.184 J/g°C)(35.0˚C)
ΔQ = 46860.8 J
q = 320 x 4.184 x 35
so this will give you your answer
Along the western coast of the United States is Death Valley, one of the hottest places in the world at the height of summertime. However just to the west is the Pacific Ocean. Death Valley runs from north to south between the Amargosa Range on the east and the Panamint Range on the west; the Sylvania Mountains and the Owlshead Mountains form its northern and southern boundaries, respectively. Using your knowledge of weather and climate and the image below, explain how a desert can form so close to an ocean.
Answers
The air is cooled by the currents, which causes it to rise and warm when it crosses land. This warming causes the air to hydrate, then afterward precipitates as the air passes deeper inland.
Can a desert and an ocean coexist?The impacts of grasslands hitting the sea are typically astounding. Namibia and or the Western Sahara constitute the place where the African desert meets north Mediterranean Sea. Moreover, the Sahara extends eastward to the Red Sea. The Atacama Desert and the Pacific Ocean meet strikingly in northern Chile.
Why do deserts surround chilly ocean currents?Cold ocean currents that move near the shore drive coastal deserts to emerge. The air is stabilised by the chilly winds, which also prevent cloud development. It produces a significant amount of fog. A dense blanket of minute water droplets which are too light to disperse as rain makes up a fog.
To know more about hydrate visit:
https://brainly.com/question/11202174
#SPJ1
Every winter i go home to michigan it takes 5 hours what is my average speed HELP!
Answers
Answer:
43.4
Explanation:
I think
What is the density of a liquid with a mass of 31.1415 g and a volume of 30.13 ml?
Answers
The liquid with a mass of 31.1415 g and a volume of 30.13 ml has a density of: 1.033 g/ml
The density formula and the procedure we will use is:
d = m/v
Where:
v= volumed= densitym= massInformation about the problem:
m = 31.1415 gv =30.13 mld= ?Applying the density formula we get:
d = m/v
d = 31.1415 g/30.13 ml
d = 1.033 g/ml
What is density?It is a physical quantity that expresses the ratio of the body mass to the volume it occupies.
Learn more about density in: brainly.com/question/1354972
#SPJ4

10 A fixed quantity of gas is placed inside a container of fixed volume at a temperature of
27°C. The gas has a pressure, P.
Which expression shows the pressure of the gas at 57°C?
A P2=(27/57) P
B
P2=(57/27) P
P2=(300/330) P
с
D
P2=(330/300) P
Answers
A gas has a pressure P at 27 °C. To calculate its pressure at 57°C we use an expression derived from Gay-Lussac's law:
C) P2 = (300/330) P
What does Gay-Lussac's law state?Gay-Lussac's law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant.
Mathematically, it can be written as:
P/T = k
A gas has a pressure P at 27 °C and we want to find the expression to calculate its pressure at 57°C.
Step 1: Convert the temperatures to the Kelvin scale.We do so by summing 273.15.
K = 27 + 273.15 = 300 K
K = 57 + 273.15 = 330 K
Step 2: Apply Gay-Lussac's law.P2/330 = P/300
P2 = (300/330) P
A gas has a pressure P at 27 °C. To calculate its pressure at 57°C we use an expression derived from Gay-Lussac's law:
C) P2 = (300/330) P
Learn more about Gay-Lussac's law here: https://brainly.com/question/24691513
what percent of the sun's energy is used by the living organism on earth
a. 1%
b. 10%
c. 20%
d. 35%
e. 50%
Answers
Hope I’m right tho
Which of the following elements can exist in more than one form in the same
state?
neon
iodine
carbon
iron
Answers
Answer:
neon
Explanation:
it comes in different colours
the molar solubility of c a ( o h ) 2 ca(oh)x2 was experimentally determined to be 0.019 m. based on this value, what is the k s p ksp of c a ( o h ) 2 ca(oh)x2 ?
Answers
The K s p of Cu(OH)2 = 3.11 * 10^-5
What is K s p value ?
The equilibrium constant for a solid material dissolving in an aqueous solution is the solubility product constant, K s p. It stands for the degree of solute dissolution in solution. A substance's K s p value increases with how soluble it is.
Solubility of A x B y salt
K s p = \(X^x Y^y S^(x+y)\)
Solubility of Ca(OH)2 = 0.0198M
K s p = 1 * 2^2 * ( 0.0198) ^ 1+2 = 3.11 * 10^-5
K s p = 3.11 * 10^-5
To know about K s p from the link
https://brainly.com/question/27964828
#SPJ4
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.9.635 ÷ 12.9 = [?]
Answers
Significant figures are the number of digits in a number. To count the significant figures we must take into account that the zeros before the digit should not be counted, but the intermediate zeros or after the digit should be counted.
Now, when we do the two-digit division, the significant figures of the quotient will be equal to those of the digit with the fewest number of significant figures. Let's see what are the figures of the terms of the division and the final result:
We see that the quotient of the division is 0.746899..., but we have to write the answer only with 3 significant figures. If the number after the last figure to which we want to approximate is greater than 5, we must add 1 to the last figure, so the result will be: 0.747.
Answer: 0.747

