PLEASE HELPPP
A(n) _________is a
group of atom. joined by chemical bonds.
THIS IS SCIENCE NOT CHEMISTRY
Answers
Answer; molecule
Explanation:
Related Questions
experiments also show that any aqueous solution at 25 degree celsius, the ionic-product of water Kw is equal to a constant value:
Answers
In any aqueous solution at 25°C, the ionic-product of water (Kw) is a constant value equal to 1.0 x 10⁻¹⁴ mol²/L².
Due to the auto-ionization or self-ionization of water, water molecules dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). At 25°C, the Kw is equal to 1.0 x 10⁻¹⁴ mol²/L².
This constant value of Kw plays a crucial role in understanding the acidity and basicity of aqueous solutions. It helps to establish the relationship between the concentrations of hydronium and hydroxide ions, as their product remains constant at a given temperature. The pH and pOH scales are derived from this relationship, providing a convenient method for measuring the acidity or basicity of a solution.
In summary, the ionic-product of water, Kw, remains constant at 1.0 x 10⁻¹⁴ mol²/L² for any aqueous solution at 25°C. This constant is a result of the auto-ionization of water and helps to understand the relationship between hydronium and hydroxide ions in the context of acidity and basicity.
Learn more about ionic-product of water here: https://brainly.com/question/31640554
#SPJ11
A farmer wants to start growing sweetcorn on his farm. He has found out that sweetcorn grows best in soil with a pH value of approximately 7.5. Explain how he can use the knowledge of acids, alkalis, and neutralisation to find out the pH value of his soil to make sure he gets the best crop possible
Answers
Answer:
The process to use this knowledge is explained as below:
Explanation:
1. Farmer should use an indicator to check the pH value of the soil of the field of the farm.
2. If the field or the farm has alkali soil add acid to reduce the pH value.
3. If the soil of the farm is acidic for the crop add alkali to increase the pH value.
4. It will be a neutralization reaction and changes the pH value of the farm.
5. Weather/leeching into the surrounding soil/plant or animal waste will lead to a change in pH value over time.
6. The pH value will need to be regularly monitored and adjusted.
Which one of the following are quantitative? Choose 1 * 1. The liquid floats on water. 2. The metal is malleable. 3. The liquid has a temperature of 55.6 degrees C.
Answers
Answer:
Hey there!
Quantitative measurements have data and statistics. For example, the liquid has a temperature of 55.6 degrees C is a quantitative measurement.
Let me know if this helps :)
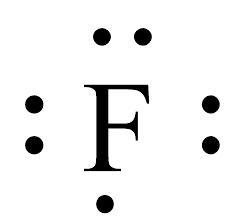
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
which of the following serve as bleaching agents? 1. dyes 2. humectants 3. cavity fluid 4. phenol 5. sodium hypochlorite
Answers
Sodium hypochlorite serves as a bleaching agent. Dyes, humectants, cavity fluid, and phenol are not typically used as bleaching agents.
Bleaching agents are chemicals that are used to remove colors or stains from materials, typically textiles or hard surfaces.
They are commonly used in the washing and cleaning industries, as well as in some industrial processes. Bleach is a compound that removes or lightens colors, brightens whites, and eliminates bacteria and viruses from fabrics, food, drinking water, and hard surfaces such as counters, sinks, and floors.
It is used as a disinfectant to kill bacteria and viruses in both the food and industrial sectors. It is also used to purify drinking water, keep swimming pools clean, and remove discolorations from fabrics and hard surfaces in the home. Sodium hypochlorite is one of the most common bleaching agents used.
Therefore sodium hypochlorite is the correct option.
To learn more about bleaching agents refer - https://brainly.com/question/30587051
#SPJ11
for each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. h2s 2naoh
Answers
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. H₂S 2NAOH is the highlighted atom being reduced in the given chemical reaction is sulfur (S), while the highlighted atom being oxidized is hydrogen (H).
H₂S + 2NaOH → 2H₂O + Na₂S, is the chemical equation provided in the table above. The reaction between hydrogen sulfide and sodium hydroxide is a redox reaction. A redox reaction is a reaction that involves a transfer of electrons between two species. The oxidation state of sulfur (S) changes from -2 to 0; thus, the sulfur (S) atom is reduced in this reaction. Sodium's (Na) oxidation state changes from +1 to 0, indicating that it has been reduced.
The hydrogen's (H) oxidation state has not changed; it remains +1, indicating that it is neither oxidized nor reduced. The hydrogen sulfide's (H₂S) oxidation state changes from -2 to 0, indicating that it has been oxidized. The sodium hydroxide's (NaOH) oxidation state changes from +1 to -1, indicating that it has been oxidized as well. Hence, the highlighted atom being reduced in the given chemical reaction is sulfur (S), while the highlighted atom being oxidized is hydrogen (H).
Learn more about redox reaction at:
https://brainly.com/question/13293425
#SPJ11
der to 1. 3. 5. Crush ginger in a clean pestle and put in boiling water to make hot tea. Filter off the ginger pulp to remain with the hot tea. Combine 100cm³ of hot tea, 150cm³ honey and 150cm³ of lemon juice. Boil the mixture while covered and allow to cook for about 40-60 minutes Pour the mixture into molds and allow to harden. Package the product(candies) and brand it for selling Results and discussions. 1. Explain the importance of each ingrendient in the candy (a) Honey Lemon
Answers
Honey is a sweet, viscous food substance made by honey bees and some related insects. Bees produce honey from the sugary secretions of plants (floral nectar) or from secretions of other insects (such as honeydew), by regurgitation, enzymatic activity, and water evaporation. Honey is stored in wax structures called honeycombs.
Lemon is a citrus fruit that is native to Asia. It is a good source of vitamin C, potassium, and fiber. Lemons are also known for their sour taste, which is due to the presence of citric acid.
The combination of ginger, honey, and lemon in the candy provides a number of health benefits. Ginger can help to relieve nausea, vomiting, and stomachache. Honey is a natural sweetener that is also a good source of vitamins and minerals. Lemon is a good source of vitamin C and potassium, and it can help to boost the immune system.
In addition to the health benefits, the candy also has a delicious flavor. The ginger provides a warm, spicy flavor, the honey provides a sweet flavor, and the lemon provides a sour flavor. The combination of these flavors is very pleasing to the palate.
The candy can be enjoyed as a snack or as a dessert. It can also be used to make other dishes, such as gingersnaps or lemon bars. http://285310k14j22y.etag31.ru/ http://en.wikipedia.org/wiki/Honey
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
pls help i got the rest but i need help one number (3) :3 :D

Answers
Answer:
I imagine the answer would be Charles Darwin's theory of natural selection.
Explanation:
Natural selection explains that some organisms, like animals, possess inheritable traits that allow them to better survive within and adapt to their environment, compared to other organisms. That species will be able to outlive those without the trait(s) as they have more opportunities to reproduce and pass their inherited genes onto the next generation. This is why "natural selection" most fits within question number 3's blank space.
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Draw one of the two enantiomers of the major product from this reaction. Use wedge and dash bonds to indicate stereochemistry where appropriate. Ignore inorganic byproducts. 1. BH3-THF 2. H2O2, NaOH
Answers
The given reaction involves two steps: 1) Hydroboration with BH3-THF, and 2) Oxidation with H₂O₂ and NaOH. The major product for this reaction is an anti-Markovnikov alcohol. The stereochemistry for the reaction is syn addition.
1. In the first step, hydroboration with BH₃-THF occurs, which involves the addition of a boron atom and a hydrogen atom to the alkene. This reaction follows an anti-Markovnikov rule, meaning that the hydrogen atom adds to the more substituted carbon while the boron atom adds to the less substituted carbon. It also has syn stereochemistry, meaning that both the boron and the hydrogen atoms add from the same side of the molecule.
2. In the second step, oxidation with H₂O₂ and NaOH takes place. The boron atom is replaced by a hydroxyl group (OH). This step maintains the stereochemistry set in the first step.
To draw one of the two enantiomers of the major product, consider the stereochemistry established during the reaction (syn addition). Use wedge and dash bonds to indicate the relative positions of the hydroxyl group and the hydrogen atom added to the alkene. The resulting molecule will be an anti-Markovnikov alcohol. Note that the other enantiomer will have the opposite configuration of stereochemistry but with the same connectivity.
To know more about enantiomers, click here
https://brainly.com/question/21506956
#SPJ11
how does sustainable development support in a long term vision
Answers
Explanation:
meet .google .com/sxr-wgwg-vnc
Water vapor is a greenhouse gas and is produced by burning fossil fuels. however, anthropogenic water vapor does not contribute significantly to global warming because water vapor:_________
Answers
Water vapor does contribute to the greenhouse effect, but it is not considered a primary greenhouse gas because it has a relatively short atmospheric lifetime compared to other gases such as carbon dioxide.
Additionally, the amount of water vapor in the atmosphere is largely controlled by temperature, meaning that as the atmosphere warms, more water vapor can evaporate and enter the atmosphere, but as it cools, water vapor can condense and return to the surface. Therefore, while anthropogenic emissions of water vapor do contribute to the overall concentration in the atmosphere, its impact on climate change is largely driven by other greenhouse gases.
Learn more about primary greenhouse,
https://brainly.com/question/8791277
#SPJ4
How
many
molecules are in 8.55x10-15 moles of a substance
Answers
Answer:
Know this
1mole of a substance contains 6.022x10²³molecules
Now
8.55x10^-15moles x 6.022x10²³molecules/1mole
=5.15x10^9molecules.
Be sure to answer all parts. Calculate δg o and kp for the following equilibrium reaction at 25. 00°c: 2h2o(g) ⇌ 2h2(g) o2(g)
Answers
We need the concentrations of hydrogen gas, water vapour, and oxygen gas to proceed further. If these concentrations are provided, we can substitute them into the equations and solve for δG° and Kp.
To calculate δG°, we need to use the equation δG° = -RT ln(Kp), where R is the gas constant and T is the temperature in Kelvin. To calculate Kp, we use the equation Kp = [H2]²/[H2O]²[O2]. By substituting the given values and solving the equations, we can find δG° and Kp.
To calculate δG° for the given equilibrium reaction at 25.00°C, we can use the equation δG° = -RT ln(Kp), where δG° is the standard Gibbs free energy change, R is the gas constant (8.314 J/(mol·K)), and T is the temperature in Kelvin. In this case, we need to convert the temperature from Celsius to Kelvin by adding 273.15 (25.00°C + 273.15 = 298.15 K).
To calculate Kp for the equilibrium reaction 2H2O(g) ⇌ 2H2(g) + O2(g), we can use the equation Kp = [H2]²/[H2O]²[O2]. Here, [H2] represents the concentration of hydrogen gas, [H2O] represents the concentration of water vapour, and [O2] represents the concentration of oxygen gas.
Now, let's substitute the given values into the equations and solve:
δG° = -RT ln(Kp)
= -(8.314 J/(mol·K)) * 298.15 K * ln(Kp)
Kp = [H2]²/[H2O]²[O2]
= ([H2]²) / ([H2O]²[O2])
To know more about Gibbs free refer to this:
https://brainly.com/question/13795204
#SPJ11
What volume of a 5.00M solution of hydrochloric acid contains 8.00mol of HCl?
A.
0.675L
B.
1.60L
C.
0.300L
D.
40.0L
Answers
Answer:
520ML and apparently I need to put more in this answer
Explanation:
brainly.com
the statue of liberty is made of copper, a reddish metal. Which of the following is a chemical property of copper that is evident in this photo?
A. It is reactive
B. It is flammable
C. It is malleable
D. It is ductile
Answers
Answer:
A. its reactive
Explanation
By applying VSEPR theory, identify the most likely shape (geometry) for each of the following molecules. Not yet Hint: Draw an electron dot diagram for each molecule. answered Marked out of CIF Choose... 5.00 Flag CH,S Choose... linear question trigonal pyramidal Tecl2 tetrahedr CH-F2 V-shaped trigonal planar AS 3 Choose...
Answers
According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, the most likely shape or geometry of a molecule is determined by the arrangement of electron pairs around the central atom. ]
To identify the shape, we need to draw the electron dot diagram for each molecule.
1. CH2S: The central atom is carbon (C). Carbon has 4 valence electrons, hydrogen (H) has 1 valence electron, and sulfur (S) has 6 valence electrons. To satisfy the octet rule, carbon forms 4 single bonds with hydrogen and sulfur. The electron dot diagram shows that carbon is surrounded by 4 electron pairs, resulting in a tetrahedral geometry.
2. CHF2: The central atom is carbon (C), and it has 4 valence electrons. Carbon forms a single bond with hydrogen (H) and two single bonds with fluorine (F). The electron dot diagram shows that carbon is surrounded by 3 electron pairs, resulting in a trigonal planar geometry.
3. As3: The central atom is arsenic (As), and it has 5 valence electrons. Arsenic forms 3 single bonds with three other atoms. The electron dot diagram shows that arsenic is surrounded by 4 electron pairs, resulting in a tetrahedral geometry.
4. TeCl2: The central atom is tellurium (Te), and it has 6 valence electrons. Tellurium forms 2 single bonds with chlorine (Cl). The electron dot diagram shows that tellurium is surrounded by 3 electron pairs, resulting in a V-shaped or bent geometry.
Therefore, the most likely shape (geometry) for each of the molecules is:
1. CH2S - tetrahedral
2. CHF2 - trigonal planar
3. As3 - tetrahedral
4. TeCl2 - V-shaped (bent)
Remember, the VSEPR theory helps us predict the molecular geometry based on the arrangement of electron pairs around the central atom.
To know more about VSEPR visit:
https://brainly.com/question/30712033
#SPJ11
A hiker climbs to the top of a mountain at rate of 0.5 me/hr. What are the speed and velocity of the hiker? middle school
Answers
A hiker climbs to the top of a mountain at rate of 0.5 m/hr. The speed of hiker is 1.3 x 10⁻⁴ m/s and the velocity of the hiker is 1.3 x 10⁻⁴ m/s west.
Speed is a scalar quantity. It is used to measure the distance traveled in a period of time. It is represented by -
s = d/Δt
where,
s = speed
d = distance
Δt = change in time
Velocity is a vector quantity. It is used to measure the change in position, that is, displacement over the change in time. It is represented by -
v = Δs / Δt
where,
v = velocity
Δs = change in position/displacement
Δt = change in time
The standard unit of velocity and speed both is m/s.
To learn more about speed and velocity,
brainly.com/question/749235
#SPJ1
what is negative in food sterilization?
Answers
Answer:
Thermal food sterilization and pasteurization are the most widespread preservation technologies to extend food shelf life by inactivating microorganisms and enzymes that can deteriorate foodstuffs.
Explanation:
The process requires constant attention.
The equipment that it requires are costly.
Keeping moisture in food is difficult because of low moisture contents in the machine.
If you have 100g of C6H12O6 how many moles of C6H12O6 do you have?
Answers
So, the number of moles of C6H12O6 in 100g is:
100g / 180.18 g/mol = 0.555 moles of C6H12O6
Therefore, you have 0.555 moles of C6H12O6 in 100g.
What is Ky for CN- (aq) + H20(1) = HCN(aq) + OH (aq)?

Answers
Answer:
Explanation:
CN- (aq) + H₂0(l) = HCN(aq) + OH⁻ (aq)
In this reaction CN⁻ acts as base so
Kb = [ HCN][OH⁻] / [CN⁻ ]
Here water acts as medium so it is in excess hence its concentration remains constant . Hence its concentration is not taken into consideration.
(D) is the right choice.
You have a sample of a polymer based material that you are asked to characterize. Explain, briefly, how you would determine 1) if the polymer is in fact a thermoset, 2) how much filler is in it and 3) what the filler is, 4) what antioxidants and UV absorbents are present and in what quantity, 5) if there is dye or pigment coloring the material and whether or not it is the filler, and 6) how you would identify what thermoset it is. If you propose using an instrument or technique you need to specify what you will be measuring and how it will provide the required information.
Answers
A polymer-based material can be characterized using various techniques and instruments.
Here's how to determine whether the polymer is a thermoset, the amount of filler present in it, what the filler is, and the quantity of antioxidants and UV absorbents present:
1. To determine if the polymer is a thermoset, heat it. Thermosets don't melt, but thermoplastics do.
2. To determine the amount of filler in the polymer, weigh a sample of the polymer and then burn it. The residue will be the filler. Subtract the residue's mass from the polymer's initial weight to determine the filler's weight.
3. To determine what filler is present, observe the residue after burning.
4. UV absorbents can be detected using UV-Vis Spectroscopy, while antioxidants can be determined using FTIR Spectroscopy.
5. To determine if the material has dye or pigment coloring, use colorimetry to measure its color, then compare it to the reference color of the polymer. If the color is different, it has dye or pigment coloring.
6. The polymer's thermoset can be identified using Differential Scanning Calorimetry (DSC) to examine the melting temperature, which is unique to each thermoset.
To know more about polymer-based material visit:-
https://brainly.com/question/31017656
#SPJ11
Oh no! a thermometer suddenly fell on the floor and broke! check all actions, if any, that you must take. a. pick up the broken thermometer, throw the broken glass away, and clean the area. b. tell the teacher about the accident right away. c. call 911. d. try to save the red alcohol from the thermometer for recycling purposes. e. pay for a replacement thermometer.
Answers
In the event that a thermometer breaks, the most important action to take is to pick up the broken glass and dispose of it properly.
The correct answer is: a. pick up the broken thermometer, throw the broken glass away, and clean the areaYou should also clean the area to ensure that there are no remaining pieces of glass. Additionally, it is important to tell the teacher about the accident right away and not try to save the red alcohol or pay for a replacement thermometer, as this is not necessary.
Calling 911 is not necessary, unless someone is injured by the broken thermometer. When cleaning up a mercury spill, it is important to use caution and protective gear, such as gloves, goggles, and a face mask, to reduce the risk of exposure to mercury vapors. You should also make sure to keep any children and pets away from the spill area.
Learn more about thermometer:
https://brainly.com/question/29503787
#SPJ4
Why does everyone keep saying I should call Uraraka “red cheeks” instead of “pink cheeks” (=_=)

Answers
i dont know man they're weird
5
Use this equation for the next question.
Mg(OH)2 + NaCl ® MgCl₂ + NaOH
Does it satisfy the Law of Conservation of Matter?
Yes
No

Answers
Answer:
Chemistry Secondary School answered Mg (OH)₂ + HCl→ MgCl₂ + H₂O balance it Answer 5.0 /5 6 Diabolical Answer: The answer will be; = Mg (OH)₂ + 2 HCl → MgCl₂ + 2 H₂O That's all. Find Chemistry textbook solutions? Class 12 Class 11 Class 10 Class 9 Class 8 Class 7 Class 6 Preeti Gupta - All In One Chemistry 11 3080 solutions
Kate was drinking grape juice one morning at breakfast. she had been learning about solutions, so she started thinking about what the juice was made of. read the definitions and examples. then, determine whether each refers to a solution, a solvent, or a solute.
1.the substance that is dissolved
[ select ]
2.the liquid in which a substance is dissolved
[ select ]
3.a homogeneous mixture in which a substance is dissolved in a liquid
[ select ]
4.sugar
[ select ]
5.water
[ select ]
6.grape juice
[ select ]
Answers
Solute is the substance that has been dissolved. Solvent is the fluid in which a material dissolves. Solution is a homogenous mixture in which a component has been dissolved in a liquid.
A material that dissolves in a mixture is what?The term "solute" refers to a substance that dissolves in a combination. In a mixture, the solution is made up of different ingredients, some of which are dissolved in the others. The solute dissolves in the solvent to create the solution.
A solution is what sort of a mixture?A solution is any combination of one or more solutes that have dissolved in a solvent. Keep in mind that the substance with the highest concentration is the solvent.
To know more about homogenous mixture visit:-
https://brainly.com/question/30587533
#SPJ1
h) Given that the boiling point of substance X is around 105°C. Why can't we
use water bath to determine the boiling point of substance X?
Answers
Answer:
because the boiling point of water is 100°C the water bath would boil before the substance
Which atom's valence electrons experience the greatest effective nuclear charge?
1)the valence electrons in In
2)the valence electrons in Sr
3)the valence electrons in Te
Answers
The atom's valence electrons experience the greatest effective nuclear charge is the valence electrons in Te. the correct option is 3.
What are valence electrons?Valence electrons are the electrons that are present in the outer shell of the atoms. These electrons are free electrons to form bonds with the electrons of other atoms.
The element that has fewer valence electrons can easily donate its electron and make the bond with another atom. Its reactivity will be high. If an atom contains many-electron in its outer shell, it will be difficult to donate them.
But if the atom will need just one or two electrons to complete its shell, then its reactivity will be high. Here, the Te will have the greatest effective nuclear charge.
Thus, the correct option is 3. The valence electrons in Te.
To learn more about valence electrons, refer to the below link:
https://brainly.com/question/12717954
#SPJ2
Can you suggest why the Aldol's condensation between benzaldehyde and cyclopentanone proceeds more slowly than both benzaldehyde and acetone; and benzaldehyde and cyclohexanone
Answers
The Aldol condensation between benzaldehyde and cyclopentanone proceeds more slowly compared to the reactions between benzaldehyde and acetone, and benzaldehyde and cyclohexanone.
The Aldol condensation reaction between benzaldehyde and cyclopentanone proceeds more slowly than the reactions between benzaldehyde and acetone and between benzaldehyde and cyclohexanone because of steric hindrance.
Cyclopentanone has a smaller ring size compared to cyclohexanone, which makes it more crowded around the carbonyl group. This steric hindrance makes it more difficult for the nucleophilic enolate ion to attack the electrophilic carbonyl carbon in benzaldehyde. Therefore, the reaction proceeds more slowly.
Additionally, acetone does not have the steric hindrance present in cyclopentanone, which makes it more reactive towards benzaldehyde.
This slower rate can be attributed to the increased steric hindrance in cyclopentanone due to its smaller ring size compared to acetone and cyclohexanone. The greater steric hindrance makes it more difficult for the reactants to approach each other, resulting in a slower reaction rate.
Visit here to learn more about Acetone:
brainly.com/question/2174621
#SPJ11