Answers
Have a good day!
Related Questions
PLEASE HELP I HAVE THIS TEST TOMORROW AM GIVING MORE POINTS THAN I SHOULD!
An unlit match contains approximately 1,000 J of chemical energy. When it burns, the match releases thermal energy and light energy. After it has burned, the remaining match contains 250 J of chemical energy. If the thermal energy was measured to be 400 J, how much light energy did the match emit?
Answers
Answer:
match emit 350 J light energy.
Explanation:
By law of conservation of energy, total energy before and after reaction for an isolated system remains constant.
Total energy for unlit match = 1000 j
When it burns type of energy released
thermal energy = 400 j
light energy --> it value is unknown which we have to find
also remaining match has 250 joule of chemical energy
applying law of conservation of energy
energy before match was unlit = energy after match was lit
1000 j = thermal energy + light energy + chemical energy of remaining match
1000 J = 400 J + light energy + 250 J
light energy = 1000 J - 400J - 250J
light energy = 350 J
Thus, match emit 350 J light energy.
Cu + 2 H2SO4 → CuSO4 + 2 H2O + SO2
Look at the above reaction, what is the name of the acid on the REACTANT side of the equation?
Answers
Sulphuric acid
Someone pls help me I will make you brain

Answers
Answer:
more drought is not a global warming
Answer:
i think it is human migration
because when drought becomes more its one sign of global warming and when land becomes less there wont be place for people to plant trees
Does a hypothesis need to be correct for an experiment to be a success? Explain why or why not?
Answers
Answer:
No it does not need to be correct to be a success. It would just need to be reasonable and possible for you to do so that you dont hypothesize that animals are actually human because that is unlogical and impossible
Explanation:
Answer:
No, of course not.
Explanation:
The goal of a hypothesis is for the researchers to determine whether or not they accurately guessed what would happen in their experiment. The fact that the hypothesis was right or wrong has no effect other than if the researchers had a correct educated guess.
3 , from
lowest to highest: A) Exosphere; B) Stratosphere; C)
Troposphere
A
A, C, B
B
B, A, C
С
C, B, A
D
C, A, B
Answers
Answer:
troposphere
stratosphere
exosphere
The answer is C. C, B, A
rutherford and JJ thompson both contributed to what we know about the structure of the atom. What experiments did they perform and how did it change what we know about the structure of the atom? Furthermore describe the structure of an atom using only two words
Answers
Answer:
J.J. Thomson's experiments with cathode-ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. I would describe the structure of an atom as the plum pudding model.
How can you control the flow of power in a circuit?
Answers
There are multiple ways to prevent these situations from occurring. The three most common protective devices are fuses, circuit breakers, and surge protectors
Explanation:
the more massive an object is, the more
Answers
Answer:
it have more more mass, gravitational potential energy
Explanation:
A person drinks 1900g of water, H2O, per day. How many moles of water did they consume?
Answers
The person consumes approximately 105.5 moles of water per day.
To calculate the number of moles of water consumed, you'll need to use the formula:
moles = mass (g) / molar mass (g/mol)
For water (H2O), the molar mass is 18.015 g/mol (sum of atomic masses: 2 × 1.008 for H and 1 × 16.00 for O). Given that the person drinks 1900 g of water per day, you can plug the values into the formula:
moles = 1900 g / 18.015 g/mol ≈ 105.5 moles
So, the person consumes approximately 105.5 moles of water per day.
To learn more about molar mass, refer below:
https://brainly.com/question/22997914
#SPJ11
This hydrocarbon is incomplete. Draw the hydrogen atoms and the bonds connecting them to carbon atoms such that each carbon atom has four bonds. Then record the number of hydrogen atoms you drew using a text box.

Answers
The number of the hydrogen atoms that would be required from the diagram is 10.
What is a saturated compound?A Saturated compound has all its carbon atoms connected by single bonds, and each carbon atom is bonded to the maximum number of hydrogen atoms possible. This arrangement allows the compound to have no available or unsaturated bonds for additional atoms.
The compound that is shown must be butane as such the number of the hydrogen atoms that it contains is a total of ten.
Learn more about hydrocarbon:https://brainly.com/question/32019496
#SPJ1
1. Look at yourself in the front of the spoon (the side where the food sits). What do you see?
The you see the image
Answers
Answer:
If you look at yourself in the front of the spoon, you see that the mirror forms an inverted real image.
Explanation:
The word 'concave' means to bend inwards.
A curved mirror is a mirror that has a curved reflecting surface.
The front side of the spoon is just like a concave mirror. If you look at yourself in the front of the spoon, you see that the mirror forms an inverted real image.
Concave mirrors produce real inverted image of an object only if it is placed further away from the mirror than the focal point.
Answer:
It shows me upside down like a ghost. OoooOooh
Explanation:
Boo
is an example of a solution that contains a dissolved substance
Answers
Answer:
An aqueous solution is water that contains one or more dissolved substances. The dissolved substances in an aqueous solution may be solids, gases, or other liquids.
in a nucleic acid, adjacent nucleotides are bound to each other in what way?
Answers
The adjacent nucleotides are bound to each other through a phosphodiester bond in a nucleic acid.
What is nucleic acid?Nucleic acid is a biopolymer made up of nucleotide monomers that make up nucleic acid chains. The nucleotide's three components are a five-carbon sugar, a phosphate group, and a nitrogenous base. Nucleic acids are present in all living cells, including viruses and bacteria, and they play a critical role in storing, transmitting, and expressing genetic information. RNA and DNA are two types of nucleic acids.
The phosphate group in one nucleotide forms a phosphodiester bond with the hydroxyl group on the sugar molecule of the next nucleotide in line in nucleic acids. This reaction is carried out by removing a molecule of water, resulting in a strong covalent bond between two nucleotides. These bonds make up the sugar-phosphate backbone of a nucleic acid chain, which is fundamental to its structure.
Learn more about Nucleic acid: https://brainly.com/question/17701344
#SPJ11
1.Compare Endothermic reactions to exothermic reactions (Define each and list 2 characteristics for each. Fill in the table below.-ENDOTHERMIC -EXOTHERMIC•Positive H Value •Negative H •ValueAbsorbs heat •Releases Heat•Products have higher energy •Products have a lower energy
Answers
When the enthalpy is positive (H > 0), the reaction is endothermic, that is, it absorbs energy.
When the enthalpy is negative (H< 0), the reaction is exothermic, that is, it releases energy.
Therefore, the correct matches are
Endothermic:
• Positive H Value.
,• Absorbs Heat.
,• Products have higher energy.
Exothermic:
• Negative H Value.
,• Releases Heat.
,• Products have lower energy.
how to make calcium chloride conduct electricity? other than putting it in a solution?
Answers
Answer:
please make me brainalist
Explanation:
Chloride compounds can conduct electricity when fused or dissolved in water. Chloride materials can be decomposed by electrolysis to chlorine gas and the metal.
What does control group mean
Answers
Why must concentrated hydrochloric acid be added to manganese (IV) oxide slowly, a portion at a time?
Answers
Answer:
to produce hydrogen
Explanation:
dudugv. gueghhyrf. jjurdsrgjnbufurxjovezjidxhu. jhddf.
Hydrochloric acid reacts with manganese dioxide result in the evolution of chlorine gas. The HCl is added slowly to one portion at a time to collect the chlorine gas evolved with a pungent smell.
What is manganese oxide?Manganese oxide is an ionic compound between the transition metal Mn and atmospheric oxygen, where the oxidation state of Mn is 5. Transition metals shows variable oxidations states.
Manganese oxide reacts with hydrochloric acid to form manganese chloride, water and chlorine gas. This reaction is an example of displacement reaction.
Here we are using a concentrated HCl. As the concentration of reagents increases, the rate of reaction also increases. Thus if we use bulk HCl initially, more chlorine gas will be evolved with pungent smell and suffocating effects. That's why we have to add Con. HCl slowly with portion wise.
To find more on displacement reaction ,refer here:
https://brainly.com/question/29307794
#SPJ2
In order to break water into hydrogen and oxygen, water is heated to more than 500°C. Which kind of reaction is this and
why?
It is endothermic because heat needs to be absorbed by the reactants to form the products.
It is exothermic because heat needs to be released by the reactants to form the products.
It is endothermic because heat needs to be released by the reactants to form the products.
It is exothermic because heat needs to be absorbed by the reactants to form the products.
Answers
It is exothermic because heat needs to be released by the reactants to form the products.
Splitting of water moleculesWater molecule is made up of two atoms of hydrogen and one atom of oxygen. That is H2O.
An exothermic reaction is the reaction in which energy, in the form of heat, is released into the environment. Some examples of exothermic reaction are:
Splitting of water moleculesRusting of iron andBurning of sugar.Splitting of water molecule involves a process called thermal dissociation reaction which uses high temperature to split water molecules into its two components such as hydrogen and oxygen.
Here, the reactants absorbs the heat which is needed to form the products.
Learn more about exothermic reaction here:
https://brainly.com/question/13892884
when melted iron solidifies without any change in temperature, what is happening on the atomic level? a. the iron atoms are gaining kinetic energy. b. the iron atoms are losing kinetic energy. c. the iron atoms are gaining potential energy. d. the iron atoms are losing potential energy.
Answers
The correct answer to the question is option B: the iron atoms are losing kinetic energy and potential energy when melted iron solidifies without any change in temperature.
When melted iron solidifies without any change in temperature, the iron atoms are losing kinetic energy, and they are losing potential energy as well.
During the process of melting, the iron atoms absorb energy, which makes them move more rapidly, and this increased kinetic energy enables them to overcome the intermolecular forces that hold them together in the solid state. As the temperature decreases, the kinetic energy of the iron atoms decreases, and eventually, they are no longer able to overcome these intermolecular forces. As a result, they begin to settle into a regular crystal lattice, and the iron solidifies.
At the same time, as the iron atoms settle into the crystal lattice, they release potential energy, which is stored in the bonds between the atoms. As the atoms become more tightly packed in the solid state, this potential energy is converted into kinetic energy, which causes the iron atoms to vibrate more slowly.
Learn more about potential energy here:
https://brainly.com/question/24284560
#SPJ11
What is the potential energy of the reactants?
a.) 20kJ
b.)40kJ
c.)60kJ
d.)80kJ
e.)100kJ
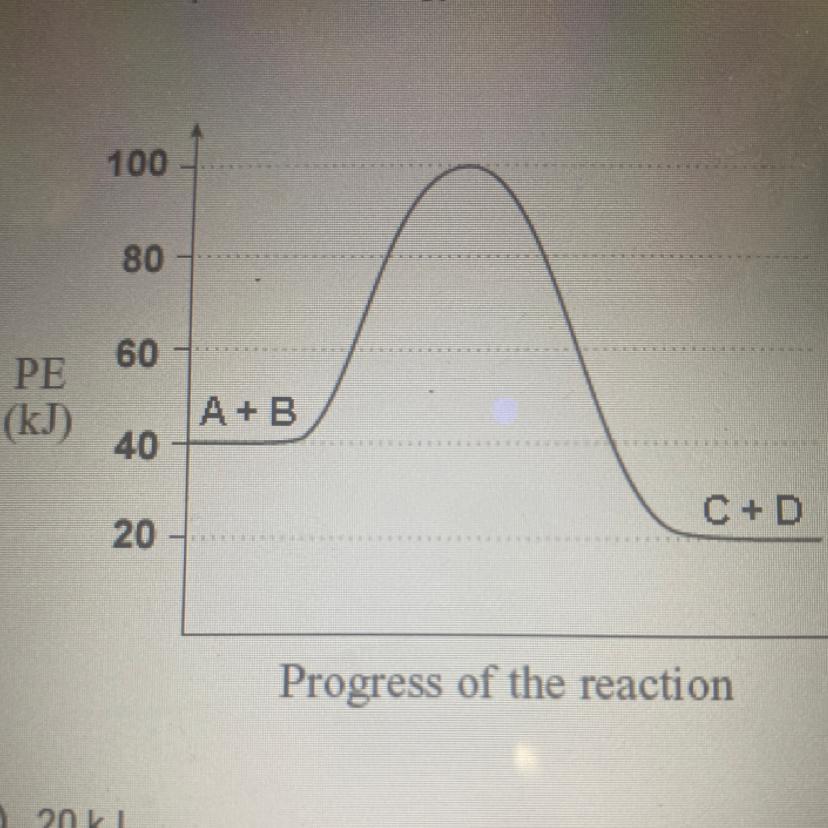
Answers
The given plot represents the change in energy of the system with respect to the progress of the reaction for an exothermic reaction. The potential energy of the reactants is 40 kJ. The correct option is B.
What is an exothermic reaction?The chemical reactions which proceed with the evolution of heat energy are called exothermic reactions. In an exothermic reaction the system loses heat to the surroundings. So 'q' and ΔH will be negative.
Since heat is given out in exothermic reactions, the enthalpy of the products will be less than that of the reactants. Here the potential energy of the reactants is 40 kJ.
Thus the correct option is B.
To know more about exothermic reaction, visit;
https://brainly.com/question/28546817
#SPJ9
What pairs of aqueous solutions form percitipate when mixed?
Answers
When silver nitrate and sodium chloride are combined with water, silver chloride will solidify and precipitate out of solution. In this instance, silver chloride is the precipitate.
Which four liquid precipitation examples are there?Precipitation includes the following: rain, hail, sleet, and snow. Rain forms when water vapour in clouds condenses on dust particles, which eventually grow too big to stay in the cloud and fall to the ground, where they collect more water and enlarge further.
What does the precipitation reaction in aqueous solution look like as an example?The chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride precipitated out, is among the greatest examples of precipitation reactions. This precipitation reaction resulted in the formation of an insoluble salt.
To know more about precipitate visit:-
https://brainly.com/question/11387485
#SPJ9
Which has a higher ionization energy: chlorine (Cl) or magnesium (Mg)? Why? Select the best answer
Cl; it takes less energy to add an electron to a valence shell that is nearly full.
Mg; magnesium has more valence electrons than chlorine.
Mg; it takes more energy to pull two electrons away from magnesium(Mg) than it does to take them from chlorine(Cl).
Cl; it takes much more energy to pull an electron away from a valence shell that is nearly full.
Answers
Removal of the loosely bounded electron is defined by the ionization energy. Chlorine needs less energy to add electrons and has higher ionization power. Thus, option A is correct.
What is ionization energy?The ability of an electron to accept or give electrons to another element in a chemical reaction by forming and creating bonds and the positive and negative charges has been defined by the ionization energy.
Magnesium has lower ionization energy than chlorine due to its large size and smaller nuclear charge. On the other hand, chlorine can easily add electrons to the valence shell which is almost full.
Therefore, option A. chlorine has higher ionization energy and can add electrons with minimum energy.
Learn more about ionization energy here:
https://brainly.com/question/6842791
#SPJ1
A man's speed is 50 m/s and his mass is 120 kg. What is his kinetic energy just before running back up? |
kg*m/s
Answers
Answer:
150,000 Joules
Explanation:
KE= 1/2 *mv^2
1/2 * 120 *50^2
60*2500
150,000 Joules
Hope this helps :)
incandescent lamps use poor conductors that become hot from ? and glow red or even white hot.
Answers
Incandescent lamps function by using poor conductors, specifically a tungsten filament, that become hot due to electrical resistance.
They emit light as a result of this. When an electric current is passed through the filament, the electrons encounter resistance as they move, which generates heat. As the filament's temperature increases, it starts to emit visible light through a process called incandescence.
This phenomenon occurs because materials at high temperatures release energy in the form of electromagnetic radiation. In the case of incandescent lamps, the heat causes the tungsten filament to glow red or even white-hot, depending on the lamp's operating temperature. The light emitted by the filament ranges from warm, yellow tones to cooler, white hues, depending on the lamp's design and power.
While incandescent lamps have been widely used for many years, they are known to be energy inefficient. This is because the majority of the electrical energy consumed by the lamp is emitted as heat rather than light. As a result, more energy-efficient alternatives, such as compact fluorescent lamps (CFLs) and light-emitting diode (LED) bulbs, have been developed and are gradually replacing incandescent lamps in various applications. These modern alternatives are designed to produce more light using less energy, reducing energy consumption and contributing to a more sustainable future.
Learn more about tungsten filament here:
https://brainly.com/question/13444614
#SPJ11
1. A sodium ion has 11 protons and 10 electrons. What is its charge?
Answers
+1
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. It is now referred to as a sodium ion.
the bond angle marked a in the following molecule is about . question 24 options: a) 109.5 b) 180 c) 60 d) 120 e) 90
Answers
The bond angle marked a in the following molecule is about 120. The atomic configuration of a molecule in three dimensions is known as molecular geometry.
Bond lengths, bond angles, torsional angles, and any other geometrical factors that affect each atom's position are all included in this, along with the molecule's overall form. In a complex molecule or ion, the angle between two orbitals that contain bonding electron pairs, or bonds, that are arranged around the centre atom is referred to as the bond angle.
A molecule is a collection of two or more atoms bonded together by the attractive forces known as chemical bonds; depending on the context, this definition may or may not contain ions. The number of atoms that make up a molecule might vary. If there are multiple atoms, they may all be the same atom or they may all be different.
the complete question is:
The bond angle marked a in the following molecule (H4C2O2N) is about.
learn more about bond angle here
https://brainly.com/question/14089750
#SPJ4
Que condiciones debo tener en cuenta antes de empezar a formar compuestos inorganicos
Answers
Knowing the physical and chemical properties of the elements being used. This is essential in order to understand how people will react and behave under certain circumstances.
It's crucial to keep in mind the following conditions before starting to create artificial computations:
Having knowledge of reaction chemistry and estequiometry. Understanding how to combine the components and the appropriate ratios to use them in order to create robust computations is crucial.
Having suitable laboratory conditions. It is essential to have access to the tools and materials needed to carry out the computation analysis and safely handle the substances.
Being aware of the effects that synthetic materials may have on the environment. It's vital to take precautions to lessen the influence of the substances use's environmental effects.
Learn more about Elements
https://brainly.com/question/24407115
#SPJ4
two reasons why emerging diseases are especially harmful to humans. (is science)
Answers
Answer:
Many emerging diseases arise when infectious agents in animals are passed to humans (referred to as zoonoses). As the human population expands in number and into new geographical regions, the possibility that humans will come into close contact with animal species that are potential hosts of an infectious agent increases.
Explanation:
Perform the following operation and express the answer in scientific notation 8.6500x10^3+6.5500x10^5
Answers
Taking into account the scientific notation, the result of the sum is 6.6365×10⁵.
Scientific notationFirst, remember that scientific notation is a quick way to represent a number using powers of base ten.
The numbers are written as a product:
a×10ⁿ
where:
a is a real number greater than or equal to 1 and less than 10, to which a decimal point is added after the first digit if it is a non-integer number.n is an integer, which is called an exponent or an order of magnitude. Represents the number of times the comma is shifted. It is always an integer, positive if it is shifted to the left, negative if it is shifted to the right.Sum in scientific notationYou want to add two numbers in scientific notation. It should be noted that when the numbers to be added do not have the same base 10 exponent, the base 10 power with the highest exponent must be found.
In this case, the highest exponent is 5.
Then all the values are expressed as a function of the base 10 exponent with the highest exponent. In this case: 8.6500×10³= 0.086500×10⁵
Taking the quantities to the same exponent, all you have to do is add what was previously called the number "a". In this case:
0.086500×10⁵ + 6.5500×10⁵= (0.086500+ 6.5500)×10⁵= 6.6365×10⁵
Finally, the result of the sum is 6.6365×10⁵.
Learn more about scientific notation:
brainly.com/question/11403716
brainly.com/question/853571
#SPJ1
after separating your partition samples in the burettes, you add 3 drops of ferric chloride to the pre/post-partition salicylic acid solutions. you notice that the pre-partition solutions turned a much more vibrant purple color than their respective post-partition solutions. this suggests that:
Answers
The more vibrant purple color of the pre-partition solution suggests that the concentration of salicylic acid is higher in the pre-partition solution than in the post-partition solution because of the complex formed.
Ferric chloride (orange in color) interacts with phenolic hydroxy groups by creating a complex that has a strong purple color. The intensity of the color is proportional to the concentration of the phenol. Because salicylic acid contains a phenolic hydroxy group, its solution will also turn purple upon the addition of ferric chloride. If the pre-partition sample has a more vibrant color, then this means that the concentration of salicylic acid in it is significantly higher, which can then inform us on the effectiveness of the separation and partition process.
You can learn more about salicylic acid here:
brainly.com/question/14635153
#SPJ4
