Answers
Related Questions
is found in all living things which make them organic.
Answers
In terms of chemicals, Carbon is the living thing in all organisms. Without carbon, there would be no life.
HELP
1) A 400g sample of alcohol (c = 2.43 J/g°C) at 16°C is mixed with 400g
of water (c = 4.19 J/g°C) at 85°C. What is the final temperature of the
mixture?
Answers
Answer:
Explanation:
Given data:
Mass of alcohol = 400 g
Specific heat capacity of alcohol = 2.43 J/g°C
Initial temperature of alcohol = 16°C
Mass of water = 400 g
Specific heat capacity of water = 4.19 J/g°C
Initial temperature of water = 85°C
Final temperature of mixture = ?
Solution:
Equation:
m₁c₁ (T₂-T₁ ) = m₂c₂(T₂-T₁)
by putting values,
400 × 4.19 × (T₂ - 85°C) = 400 × 2.43 × (T₂ - 16°C)
1676 × (T₂ - 85°C) = 972 × (T₂ - 16°C)
Find the theoretical oxygen demand for the following
solutions:
a. 200 mg/L of octanol,
CH3(CH2)7OH
b. 90 mg/L of acetone, C3H6O
Please explain steps
Answers
To find the theoretical oxygen demand for the given solutions, we need to calculate the amount of oxygen required to completely oxidize the organic compounds present in each solution.
This can be determined by using the stoichiometry of the balanced chemical reactions representing the oxidation of the organic compounds.
a. Octanol (CH3(CH2)7OH)
The balanced chemical equation for the oxidation of octanol is as follows:
2C8H18 + 25O2 -> 16CO2 + 18H2O
From the balanced equation, we can see that for every 2 moles of octanol (C8H18), 25 moles of oxygen (O2) are required.
Given that the concentration of octanol is 200 mg/L, we can convert it to moles per liter:
200 mg/L * (1 g / 1000 mg) * (1 mol / molar mass of octanol)
Next, we can calculate the theoretical oxygen demand:
Oxygen demand = (moles of octanol) * (25 moles of oxygen / 2 moles of octanol)
b. Acetone (C3H6O)
The balanced chemical equation for the oxidation of acetone is as follows:
C3H6O + 4O2 -> 3CO2 + 3H2O
From the balanced equation, we can see that for every 1 mole of acetone (C3H6O), 4 moles of oxygen (O2) are required.
Given that the concentration of acetone is 90 mg/L, we can convert it to moles per liter:
90 mg/L * (1 g / 1000 mg) * (1 mol / molar mass of acetone)
To learn more about compounds
https://brainly.com/question/26556885
#SPJ11
3. A 134.50 g sample of aspirin is made up of 6.03 g of hydrogen, 80.70 g of carbon, and 47.77 g of oxygen. What is the percent by mass of each element in aspirin?
Answers
Answer:
The percentage of mass of hydrogen in aspirin is 4.48%
The percentage of mass of carbon in aspirin is 60%
The percentage of mass of oxygen in aspirin is 35.5%
Explanation:
Given that,
Mass of sample of aspirin = 134.50 g
Mass of hydrogen = 6.03 g
Mass of carbon = 80.70 g
Mass of oxygen = 47.77 g
We need to calculate the percentage of mass of hydrogen in aspirin
Using formula for percentage of mass
\(\text{percentage of mass of hydrogen}=\dfrac{m_{h}}{m_{s}}\times100\)
Put the value into the formula
\(\text{percentage of mass of hydrogen}=\dfrac{6.03}{134.50}\times100\)
\(\text{percentage of mass of hydrogen}=4.48\%\)
We need to calculate the percentage of mass of carbon in aspirin
Using formula for percentage of mass
\(\text{percentage of mass of carbon}=\dfrac{m_{c}}{m_{s}}\times100\)
Put the value into the formula
\(\text{percentage of mass of carbon}=\dfrac{80.70}{134.50}\times100\)
\(\text{percentage of mass of carbon}=60\%\)
We need to calculate the percentage of mass of oxygen in aspirin
Using formula for percentage of mass
\(\text{percentage of mass of oxygen}=\dfrac{m_{o}}{m_{s}}\times100\)
Put the value into the formula
\(\text{percentage of mass of oxygen}=\dfrac{47.77}{134.50}\times100\)
\(\text{percentage of mass of oxygen}=35.5\%\)
Hence, The percentage of mass of hydrogen in aspirin is 4.48%
The percentage of mass of carbon in aspirin is 60%
The percentage of mass of oxygen in aspirin is 35.5%
What would be the mass of 9.76 x 1022 formula units of SrCla?
Answers
Answer:
25.7 g
Explanation:
I don't know what to put here hahaha, I hope my answer helps you ^^
Which of the following conditions on Mars would be the first to kill a human who is unprotected and unassisted by life support?
A
Colder than Antarctic temperatures.
B
Low air pressure.
C
High CO2 atmosphere.
D
Excess solar radiation due to a missing magnetic field.
Answers
Answer:
D
Excess solar radiation due to a missing magnetic field.
Explanation: Solar proton events (SPEs) are bursts of energetic protons accelerated by the Sun. They occur relatively rarely and can produce extremely high radiation levels. Without thick shielding, SPEs are sufficiently strong to cause acute radiation poisoning and death.
Hope this hels
plz mark brainliest
Make up a short story to explain jays speed and acceleration change throughout his walk. Make sure to address parts A-D
Answers
Answer:
Speed of jays increases first, again increases, decreases and then increases.
Explanation:
The speed of jays increases while moving from rest to point A and no acceleration due to constant speed. From point A to point B, the speed is continues to increase and no acceleration. From point B to point C, the speed of jays decreases and acceleration is produced in the journey due to change of speed and from point C to point D, the speed of jays again increases and acceleration is produced due to increasing the speed.
Answer:
The speed of jay's walk increases first, again increases, then it decreases and then increases due to his acceleration walk during parts A-D.
Explanation:
Which of the following are things that may change during an experiment?
Answers
Answer:
The things that are changing in an experiment are called variables. A variable is any factor, trait, or condition that can exist in differing amounts or types.
Anthracite coal d) is the most abundant grade of coal e) is very soft and burns at high temperatures a) causes the most air pollution c) is very hard and burns cleanly b) has the highest sulfur content
Answers
Answer: The correct option is C ( is very hard and burns cleanly).
Explanation:
COAL is a form of rock that is made up of mostly carbon amongst other elements which includes sulphur, nitrogen, hydrogen and oxygen. There are different types of coal which include:
--> anthracite ( 90% carbon)
--> bituminous coal ( 70-90% carbon)
--> lignite ( 60- 70% carbon) and
--> peat (60 % carbon).
Anthracite is the type of coal that contains the highest carbon content ( 90% carbon). This makes it very hard and is often a times referred to as HARD COAL. Anthracite is a higher quality coal for domestic and open fire heating. This is because it contains less impurities than other type of coal and thereby making it to BURN CLEANLY avoiding atmospheric pollution.
why would molecules with aromatic substituents disrupt the formation of amyloid?
Answers
Amyloid is a type of protein aggregate that forms when proteins misfold and aggregate into β-sheet-rich fibrils that can accumulate in tissues and cause disease.
There is increasing evidence that small molecules with aromatic substituents can inhibit amyloid formation by binding to and stabilizing the native, folded state of proteins.
One potential mechanism for this effect is that the aromatic substituents of the small molecules can interact with the aromatic side chains of the amino acid residues in the protein. This interaction can stabilize the native state of the protein by reducing the energy difference between the native and unfolded states, and prevent the protein from misfolding and aggregating into amyloid fibrils.
Moreover, the presence of aromatic substituents can also interfere with the formation of β-sheet structures, which are a hallmark of amyloid fibrils. By disrupting the formation of β-sheets, the aromatic molecules can prevent the growth of amyloid fibrils and promote the formation of less harmful oligomers or amorphous aggregates.
To learn more about amyloid, click here:
https://brainly.com/question/5184265
#SPJ4
What subatomic particles determine the mass of an atom
Answers
Answer: Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons.
Explanation:
____ are designed to make necessary adjustments to place a satellite into stable orbit.
Answers
there an A B C D thing?
Explanation:
890
Answer: thrusters
Explanation: got it right
Which of the following is an indicator of a chemical reaction? CA. Changing states of matter (solid to liquid) CB. Two different compounds mixing and remaining separate CC. Increasing in temperature D. Decreasing in size
Answers
Answer:
All except 2nd one
Explanation:
States of matter changes in chemical reactionIn Exothermic reactions temperature increasesIn first order reactions or radioactive reactions size decreases because of decayAnswer:
CB. Two different compounds mixing and remaining separate is not correct!
heyy guys, so basically i need help with stoichiometric calculation I will give you 100 points just to answer all of these answers accurately with working out (ps ill mark you brainliest x), thanks. PLEASE HELPPP. I'm desperate x
3. What masses of ethanol and ethanoic acid would need to be reacted together to give 1 g of ethyl ethanoate?
C^2H^5OH + CH^3CO^2 H → CH^3CO^2C^2H^5 + H^2O
4. What mass of iron(III) oxide would need to be reduced to produce 100 tonnes of iron in a blast furnace?
Fe^2^O^3 + CO → Fe + CO^2
5. What mass of silver nitrate as a solution in water would need to be added to 5 g of sodium chloride to
ensure complete precipitation of the chloride?
AgNO^3(aq) + NaCl (aq) → AgCl (s) + NaNO^3(aq)
6. Copper(II) oxide reacts with sulphuric acid to produce copper(II) sulphate. If this is allowed to crystallise the formula of the crystals is CuSO 4 .5H 2 O. What mass of copper oxide would be needed to produce 100 g of crystals?
CuO + H^2O + H^2SO^4 = CuSO^4 .5H^2O
7. In the following reactions calculate the mass of precipitate formed from 20 g of the metal salt in each case.
a. ZnSO^4 (aq) + 2NaOH → Zn(OH)^2(s) + Na^2SO^4(aq)
b. Al^2 (SO^4 ) 3(aq) + 6NaOH → 2Al(OH)^3(s) + 3Na^2SO^4(aq)
c. MgSO^4(aq) + 2NaOH → Mg(OH)^2(s) + Na^2^SO^4(aq)
Answers
Answer:
3. The mass of ethanol required is approximately 0.522869 g
The mass of ethanoic acid required is approximately 0.68156 g
4. The mass of iron (III) oxide required is approximately 285.952.189.095 tonnes
5. The mass of silver nitrate required is approximately 14.53 grams
6. The mass of copper oxide that would be needed is approximately 31.86 grams
7. a. The mass of the precipitate, Zn(OH)₂ formed is approximately 49.712 grams
b. The mass of the precipitate, Al(OH)₃ formed is approximately 13 grams
c. The mass of the precipitate, Mg(OH)₂, formed is approximately 14.579925 grams
Explanation:
3. The 1 mole of ethanol and 1 mole of ethanoic acid combines to form 1 mole of ethyl ethanoate
The number of moles of ethyl ethanoate in 1 gram of ethyl ethanoate, n = 1 g/(88.11 g/mol) = 1/88.11 moles
∴ The number of moles of ethanol = 1/88.11 moles
The number of moles of ethanoic acid = 1/88.11 moles
The mass of ethanol = (46.07 g/mol) × 1/88.11 moles = 0.522869 g
The mass of ethanoic acid in the reaction = 60.052 g/mol × 1/88.11 moles ≈ 0.68156 g
4. 1 mole of iron(III) oxide reacts with 1 mole of CO₂ to produce 1 mole of iron
The number of moles in 100 tonnes of iron= 100000000/55.845 = 1790670.60614 moles
The mass of iron (III) oxide required = 159.69 × 1790670.60614 = 285952189.095 g ≈ 285.952.189.095 tonnes
5. The number of moles of NaCl in 5 grams of NaCl = 5 g/58.44 g/mol = 0.0855578371 moles
The mass of silver nitrate required, m = 169.87 g/mol × 0.0855578371 moles ≈ 14.53 grams
6. The number of moles of CuSO₄·5H₂O in 100 g of CuSO₄·5H₂O = 100 g/(249.69 g/mol) ≈ 0.4005 moles
The mass of copper oxide required, m = 79.545 g/mol × 0.4005 moles ≈ 31.86 grams
7. a. The number of moles of NaOH in the reaction = 20 g/(39.997 g/mol) ≈ 0.5 moles
2 moles of NaOH produces 1 mole of Zn(OH)₂
0.5 moles of NaOH will produce 0.5 mole of Zn(OH)₂
The mass of 0.5 mole of Zn(OH)₂ = 0.5 mole × 99.424 g/mol = 49.712 grams
The mass of the precipitate, Zn(OH)₂ formed = 49.712 grams
b. 6 moles of NaOH produces 2 moles Al(OH)₃
20 g, or 0.5 mole of NaOH will produce (1/6) mole of Al(OH)₃
The mass of the precipitate, Al(OH)₃ formed, m = 78 g/mol×(1/6) moles = 13 grams
c. 2 moles of NaOH produces 1 mole of Mg(OH)₂, therefore;
20 g or 0.5 moles of NaOH formed (1/4) mole of Mg(OH)₂
The mass of the precipitate, Mg(OH)₂, formed, m = 58.3197 g/mol × (1/4) moles = 14.579925 grams
Answer:
Explanation:
i will show in details how 2 do the 1st Q n u can do the rest by following the way how it is done
3. given C^2H^5OH + CH^3CO^2H → CH^3CO^2C^2H^5 + H^2O
molar ratio of ethanol, ethanoic acid and ethyl ethanoate is 1 : 1 : 1
so mass = no. of moles * molecular mass
for same no. of moles, mass / molecular mass is the same
molecular mass of CH^3CO^2C^2H^5 = 12+1*3+12+16*2+12*2+5*1 = 88
molecular mass of C^2H^5OH = 12*2+1*5+16+1 = 46
molecular mass of CH^3CO^2H = 12+1*3+12+16*2+1 = 60
1 g of ethyl ethanoate = 1/88 mole
it requires 1/88*46 = 0.5227 g of ethanol; and
1/88*60 = 0.6818 g of ethanoic acid
to react together to form 1 g of ethyl ethanoate
What is the bond angle formed by an axial atom, the central atom, and any equatorial atom?.
Answers
The bond angle formed by an axial atom is 180 degrees and equatorial atom is 90 degrees.
Above and below the core atom are the axial atoms. These are the "pyramid points" that the molecule forms. (NOTE: Bipyramidal refers to two pyramids. The pyramids should be visible in relation to the top and bottom halves of the centre atom if you look at an image. The atoms that are located in the horizontal plane of the Centre atom are known as equatorial atoms. (Consider how close to the Earth the equator is.)
Assuming that the EX5 is made up entirely of atoms, let's continue our debate (and not lone pairs). The equatorial atoms have 120 degree binding angles with one another. The axial and equatorial atoms' bond angles are 90 degrees.
Learn more about Bond angle here:
https://brainly.com/question/14089750
#SPJ4
Determine the bond order from the molecular electron configurations. 1. (1)2(1*)2(2)2(2*)2(2p)4
and 2. (1)2(1*)2(2)2
Answers
The bond order for this molecular electron configuration is 0.
(1)2(1*)2(2)2(2*)2(2p)4:
In this electron configuration, we have 2 electrons in the bonding molecular orbital (1) and 2 electrons in the antibonding molecular orbital (1*). Similarly, we have 2 electrons in the bonding molecular orbital (2) and 2 electrons in the antibonding molecular orbital (2*). Additionally, there are 4 electrons in the 2p atomic orbital.
To calculate the bond order, we subtract the number of antibonding electrons from the number of bonding electrons and divide the result by 2:
Bond order = [(number of bonding electrons) - (number of antibonding electrons)] / 2
Bond order = [(2 + 2) - (2 + 2)] / 2 = 0
Therefore, the bond order for this molecular electron configuration is 0.
(1)2(1*)2(2)2:
In this electron configuration, we have 2 electrons in the bonding molecular orbital (1) and 2 electrons in the antibonding molecular orbital (1*). We also have 2 electrons in the bonding molecular orbital (2) and no electrons in the antibonding molecular orbital (2*).
Calculating the bond order:
Bond order = [(number of bonding electrons) - (number of antibonding electrons)] / 2
Bond order = [(2 + 2) - 0] / 2 = 4 / 2 = 2
Therefore, the bond order for this molecular electron configuration is 2.
Click the below link, to learn more about bond order:
https://brainly.com/question/10429880
#SPJ11
Explanation of the concepts of mole ratio in stoichiometry to calculate theoretical yield. Support the concept with:an explanation of the importance of considering mole ratios in two different commercial or industrial chemical processes.provide one example including a relevant equation and calculations to support the explanation. Briefly discuss the effects of limiting and excess reagents in this reaction.
Answers
Mole ratio is the proportional amount of moles of two or more compounds in a chemical reaction, and this concept is widely used in Stoichiometry, since in this type of matter we have to be comparing initial amount of reactant and final amount of product, usually, we have to convert grams of mass into moles of the compound, and when we have to compare the number of moles of different compounds, we use mole ratio, as we can see in the example below:
A + 2 B -> X
The mole ratio between A and B is 1:2, therefore if we have 3 moles of A in the reaction, we would have 6 moles of B, and with that concept in mind, we can find the mass asked in any question.
In this process, we can have the theoretical yield of a reaction, which is how much of the product is produced from the initial amount of reactant
We have two oversimplified reactions that take in account mole ratio:
1. Formation of Ammonia:
N2 + 3 H2 -> 2 NH3, here we have the following mole ratios:
1 N2 = 3 H2
1 N2 = 2 NH3
3 H2 = 2 NH3
2. Burning of Octane, which is fuel:
2 C8H18 + 25 O2 -> 16 CO2 + 18 H2O
The mole ratios are:
2 C8H18 = 25 O2
2 C8H18 = 16 CO2
2 C8H18 = 18 H2O
25 O2 = 16 CO2
25 O2 = 18 H2O
16 CO2 = 18 H2O
Limiting and excess reactants are, as the name already implies, the reactant the will limit the amount of the other reactant undergoing the reaction, and this is found through mole ratio. The excess reactant is the reactant that will not totally react, but it will have some of it left without undergoing the reaction
what is the mass of 11.3 mole of argon?
Answers
Answer:
They will determine the mass of various common elements and compounds and convert this data into values of moles, atoms and molecules.
Explanation:
What are the end products of photosynthesis? oxygen and carbohydrate water and carbon dioxide water and oxygen carbohydrate and water
Answers
The end products of photosynthesis are oxygen and carbohydrate.
The overall balanced eqation for photosynthesis reaction:
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
CO₂ is carbon dioxide or carbon(IV) oxide
H₂O is water molecule
C₆H₁₂O₆ is carbohydrate
O₂ is oxygen molecule
Light energy is transformed into a chemical energy, that is stored in a carbohydrate molecules (glucose), which are synthesized from carbon dioxide and water.
Plants (not animals) convert a solar energy into the chemical energy of sugars (food).
The pigment chlorophyll absorbs one photon and loses one electron (it passed throw an electron transport chain) in the light-dependent reactions.
More about photosynthesis: brainly.com/question/19160081
#SPJ4
3. A compound contains 15.9% aluminum, 27.4% phosphorus, and 56.6% oxygen. Calculate the empirical formula.
Answers
\(AlP_2O_6\) is the empirical formula for the substance.
The simplest whole number ratio of atoms from each component element is the empirical formula for a compound. Find the moles of each element present in the compound so that we may derive the empirical formula. To do this, we need to convert the percentages of each element to the amount in moles.
First, convert the percentages of each element to grams. Since the percentages add up to 100%, we can use 100 grams as the total mass of the compound:
Aluminum: 15.9% × 100 g = 15.9 g
Phosphorus: 27.4% × 100 g = 27.4 g
Oxygen: 56.6% × 100 g = 56.6 g
Next, convert the mass of each element to moles by dividing by the molar mass of each element:
Aluminum: \(\frac{15.9 g }{ 26.98 g/mol }= 0.59 mol\)
Phosphorus: \(\frac{27.4 g }{30.97 g/mol} = 0.89 mol\)
Oxygen: \(\frac{56.6 g }{ 16.00 g/mol} = 3.53 mol\)
Now, divide each mole value by the smallest value (0.59 mol):
Aluminum : \(\frac{ 0.59 mol }{0.59 mol} = 1\)
Phosphorus: \(\frac{0.89 mol }{ 0.59 mol }= 1.5\)
Oxygen : \(\frac{ 3.53 mol}{ 0.59 mol} = 5.97\)
To get the simplest whole number ratio, we need to round each ratio to the nearest whole number.
Hence, the compound's empirical formula is \(AlP_2O_6\).
learn more about empirical formula Refer:brainly.com/question/14044066
#SPJ1
If I add 26 mL of water to 172 mL of a 4 M NaOH solution, what will the molarity of the diluted solution be?

Answers
198 ml To create 4% NaOH, you will need 7.92 grammes of sodium hydroxide, or (4 x 198)/100. So, 7.92 grammes of sodium hydroxide are dissolved in 198 ml of water.
Does dilution lessen the molarity of a solution?The molarity of a solution is decreased by dilution. An aqueous solution's volume grows with the addition of more water, while the solute's moles remain constant. As a result, the solution's concentration and molarity are reduced.
What occurs as dilution increases?Dilution happens as the solution's volume increases. As just a result, conductivity decreases and ions per millilitre increase. The molar conductivity is calculated using one mole of ions. The molar conductivity of the solution rises as a result of increased ion separation and mobility.
To know more about hydroxide visit:
https://brainly.com/question/4251554
#SPJ1
Which compound has both covalent and ionic bonds? pls give the explanation thx :)
A NH3
B Na2SO4
C CH3COOH
D NaCl
Answers
Answer:
B.) Na₂SO₄
Explanation:
Covalent bonds are bonds which involve electrons being shared between two atoms. These bonds only occur between nonmetals.
Ionic bonds are bonds which involve electrons being given or taken by two atoms. These bonds form between a metal and a nonmetal.
NH₃ contains nitrogen and hydrogen, two nonmetals. Therefore, this molecule only contains covalent bonds.
Na₂SO₄ has both covalent and ionic bonds. The covalent bonds are found within the polyatomic ion, SO₄²⁻. Sulfur and oxygen are both nonmetals, thus covalently combining. The ionic bond forms between the Na⁺ and SO₄²⁻. Sodium (Na) is a metal which wishes to give up electrons to have a complete octet. SO₄²⁻ serves as the anion which it bonds with.
CH₃COOH contains hydrogen, carbon, and oxygen. All of these are nonmetals, thus the only bonds formed are covalent.
NaCl contains sodium, a metal, and chlorine, a nonmetal. Therefore, this compound is held together by an ionic bond.
would the stars orbiting the center of the milky way behave similarly to a planet or comit orbiting a star?
Answers
Answer:
!dk if this answers your question.
Explanation:
Astronomers have spent a lot of time measuring the rotation rates of galaxies. The rates do not fall off with distance as they would if visible matter was all there was. So, unlike the solar system, where the rate falls with distance, in galaxies the rate at the edge is too high to hold together, unless there is more mass than can be seen. This was the first indication of what is now termed 'dark matter'. It exists as huge blobs of mass that envelope galaxies. What it is made of, no one knows yet.
In the calvin cycle, if nadph begins to run out, what would be the first compound to accumulate?.
Answers
Answer:
1,3-bisphosphoglycerate
Explanation:
Darwin told us that science:
saves lives
Answers
Answer:yup!!
Explanation:
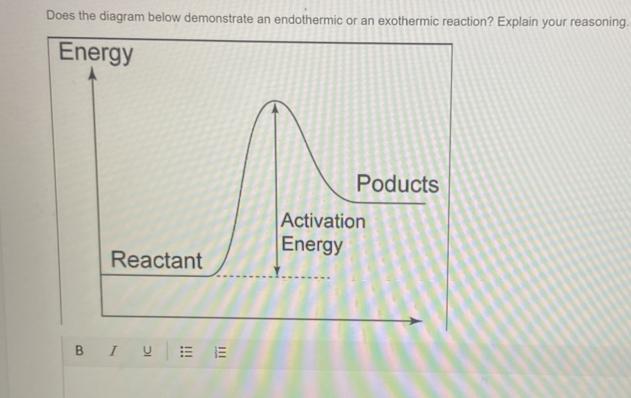
Does the diagram below demonstrate an endothermic or an exothermic reaction? Explain your reasoning.
PLEASE BE ACCURATE!!! Thank you so much!!:))
Answers
Answer:
Endothermic reaction.
Explanation:
The reactants are at a lower energy rate than the products. Because delta H is positive, energy is absorbed from the surroundings.
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
What term describes the difference of the protons and electrons in an element?
Answers
Electrons and proton are a type of subatomic particle with a negative and positive charge. Protons are held together in the atom's nucleus by the nuclear force. A subatomic particle without charge is the neutron.
What are electrons give example?The electron, the smallest constituent part of an atom, has a negative charge. Protons and electrons are present in an atom in a neutral state in an equal number. One electron and one proton are all that the hydrogen ion has. On the other hand, the atomic nucleus possesses 92 protons, that means 92 electrons.
Where are electrons found?Electrons are present outside of the atom's nucleus, in contrast to neutrons and protons that are contained inside the nucleus at its center. Negative electrons are drawn to the positively charged nucleus so because electric charges of opposite sign attract one another.
To know more about Electron visit:
https://brainly.com/question/1255220
#SPJ13
Atmospheric pressure on the peak of Kilimanjaro can be as low as
0.20 atm. If the volume of an oxygen tank is 10.OL, at what
pressure must the tank be filled so the gas inside would occupy a
volume of 1.2 x 10'L at this pressure?
Answers
On Kilimanjaro's summit, atmospheric pressure can be as low as 0.20 atm. A tank of oxygen has a 10.0 L capacity. At this pressure, the gas inside would have a volume of 1.2 103 L.
Boyle's law states that a gas's pressure is inversely related to its volume in a container.
Additionally, if the temperature is constant, it may be said that the ratio of a gas's pressure to its volume is constant.
P1V1 = P2V2
Here, P1 denotes the air pressure at the top of Mount Kilimanjaro, V1 the capacity of the oxygen tank, P2 the required fill pressure, and V2 the volume of the gas.
P2 = 0.200 x 10/1200 x 0.002 atm P2 = 0.20 atm (10 L) = P2 (1.2 x 103 L)
Therefore, the pressure at which the tank must be filled is 0.002 atm
To know about atmospheric pressure
https://brainly.com/question/26149711
#SPJ4
The pH of a solution can be determined by using a
A. Filter paper
B. Potentiometer
C. Thermometer
D. pH paper
real answers plz not bots