Answers
Answer:
S0rRy i d0nT kn0w fRenCh
Related Questions
7.49 x 105 mm
34500 inches
0.45 miles
885 yards
smallest to largest lengths
Answers
34500
28512
31860
answer:
7.49 x 105 mm
0.45 miles
885 yards
34500 inches
reason:
I converted all the measurements to inches.
First, I multiplied 7.49x105 to get 786.45mm
Then, I divided it by 25.4 to get about 30.92 inches
Next, I converted 0.45 miles to inches by multiplying it by 63,360 to get 28,512 inches
For the yards, I took 885 and multiplied it by 36 to get 31,860 inches
Since the last one was already in inches, all I needed to do was put them in order from least to greatest
Smallest to largest lengths are:7.49×105 mm ,0.45 miles ,885 yards ,34500 inches.
How to inter-convert the length quantities?Among the length quantities comprising of millimeters,inches,miles and yards the smallest quantity is of inches.As inches is the smallest quantity and the conversion of other quantities in to inches is easier .
All the other quantities are converted in inches,
7.49×105 mm=786.45 mm
As 1 inch =25.4 mm therefore 786.45 inches /25.4 mm=30.96 inches.
As 1 mile=63360 inches
0.45 miles is equal to 63360×0.45=28512 inches.
As 1 yard=36 inches
thus,885 yard =885×36=31860 inches
As all quantities are now in inches arranging them from smallest to largest lengths
7.49×105 mm
0.45 miles
885 yards
34500 inches
To know more about inter- conversion of units click here:
https://brainly.com/question/28052913
#SPJ2
Calculate the volume
of a piece of red oak
with a mass of 25 kg
given the density of
red oak is
740 kg/m3.
Answers
Answer:
0.03 m³Explanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{25}{740} = 0.033788... \\ \)
We have the final answer as
0.03 m³Hope this helps you
Classify each statement as an observation, a law, or a theory. Drag the appropriate items to their respective bins.: (A): Neon does not react with sodium at room temperature and pressure. (B): Chlorine reacts violently with sodium at room temperature and pressure. (C): If elements are listed in order of increasing mass of their atoms, their chemical reactivity follows a repeating pattern. (D): The reactivity of elements depends on the arrangement of their electrons. Observation: (A), (B) Law: (C) Theory: (D)
Answers
Observation, theory, and law all play significant roles in the philosophy of science. The statement the reactivity of elements depends on the arrangement of their electrons is a theory.
Chlorine reacts violently with sodium at room temperature and pressure and Neon does not react with sodium at room temperature and pressure are observations.
The statement, If elements are listed in order of the increasing mass of their atoms, their chemical reactivity follows a repeating pattern = law.
In most contexts, the act of sensing the exterior real objects that surround us is known as observation. Observation relies heavily on the use of many sensory organs.
A scientific explanation of a witnessed action or occurrence is an example of what we mean when we talk about theory. It provides an explanation for the observations or events that are based upon any hypothesis that has been demonstrated to be correct. A phenomenon or behavior that has been observed can be described using the term "law." It is widely accepted in most circles as a statement of fact.
The complete question and answer are attached.
Want to know more about observation, law, and theory visit the link which is given below;
https://brainly.com/question/4782822
#SPJ4

QUESTION 1
Sugar is blank meaning it can readily absorb moisture.
Answers
Answer:
sugar is hygroscopic
Explanation:
sugar is hygroscopic
how do you calculate metal density?
Answers
Answer:
You can divide the mass by the volume to calculate the density of the metal
Explanation:
HELP IS NEEDED RIGHT NOW!!!!!!

Answers
1. Eukaryotes and Prokaryotes: I have a cell membrane (plasma membrane)
2. Eukaryotes: I have nucleus
3. Prokaryotes: Bacteria are an example of me
4. Eukaryotes: Fungi are an example of me.
5. Eukaryotes: Animals are an example of me.
6. Eukaryotes: I contain ribosomes.
7. Eukaryotes: I contain membrane-bound organelles.
8. Eukaryotes and Prokaryotes: I contain cytoplasm.
9. Eukaryotic cell: Your body cells are made of this type of cell.
10. Eukaryotes: Protists are an example of me.
11. Eukaryotes: Plants are an example of me.
12. Eukaryotes and Prokaryotes: I contain genetic material such as DNA.
What are Eukaryotes and Prokaryotes?Prokaryotes are organisms that are composed of cells that do not have a cell nucleus or any membrane-encased organelles.
Eukaryotes are organisms that are composed of cells that have a cell nucleus or any membrane-encased organelles.
An example of a prokaryote is bacteria. Fungi are an example of a eukaryote.
Learn more about prokaryotes and eukaryotes at: https://brainly.com/question/2088739
#SPJ1
I need help calculating the error % in molar mass
Answers
Error % = |(experimental - actual) / actual| x 100%
For example, let's say the actual molar mass of a compound is 100 g/mol, and the experimental molar mass determined in the lab is 95 g/mol. The error percentage would be:
Error % = |(95 - 100) / 100| x 100%
Error % = |-0.05| x 100%
Error % = 5%
Therefore, the error percentage in molar mass is 5%
Cho a g hhA (Na và Ca) vào y g H2O thì thu đc 6,72l khí (đktc) và ddB trg đó nồng độ M kiềm canxi. Tính x,y bt C% của Na là 8%
Answers
Answer:
t5gbt 4 tbg3rvb tjj5tb4b4bv
Explanation:fhtb v4bvtvhbvv bh
Jean is a crime scene investigator. She needs to draw a sketch that shows the height of the objects in the room. Which type of perspective should she draw?
Answers
One-point perspective is a type of linear perspective where all lines converge to a single vanishing point on the horizon line. This technique is particularly suitable when depicting interior spaces or scenes viewed from a single vantage point.
For Jean to accurately depict the height of objects in the room, she should utilize a one-point perspective drawing. One-point perspective is a type of linear perspective where all lines converge to a single vanishing point on the horizon line. This technique is particularly suitable when depicting interior spaces or scenes viewed from a single vantage point.
By placing the vanishing point on the horizon line, Jean can establish a point of convergence for all vertical lines in the room. The height of objects, such as furniture, walls, or doors, can be represented accurately by drawing vertical lines from their base to the vanishing point. This approach creates a sense of depth and proportion, allowing viewers to perceive the relative heights of objects within the room.
Using one-point perspective will help Jean create a clear and realistic representation of the room's spatial arrangement, enabling her to accurately communicate the height relationships between different objects present in the crime scene.
For more question on perspective
https://brainly.com/question/30088701
#SPJ8
how many atoms of hydrogen and oxygen are present in 5 gm of HNO3
Answers
ANSWER: note the amounts of atoms of all the component in HNO3, which are 1 atom of hydrogen, 1 atom of nitrogen and 3 atom of oxygen.
Evan answer it................................
Answers
Answer: answer what..
Answer:
evan..
Explanation:
How many molecules of water are in 1 liter of water
Answers
Answer:
3.34 × 1025 molecules
Water molecules
Explanation:
Water (H2O) has a molecular weight of 18 grams (g), and 1 liter (L) of water weighs 1,000 g. A molecular weight often is simply referred to as a mole. Thus, 1 L of water contains 55.6 moles of water. Multiplying by Avogadro's number we find that 55.6 moles of water contains 3.34 × 1025 molecules.
Hello I was wondering if my if my answer is correct? I also have another answer which is 0.063

Answers
First of all finding the pressure, again we will be assuming an ideal pressure of 1 atm, this will be our total pressure, now we have to find the partial pressure for H2. The partial pressure for H2O is related to its temperature, when it's 40°C we will have a pressure of 55.37 torr, which is 0.0728 atm
Ptotal = Ph2 + Ph2o
1 atm = Ph2 + 0.0728 atm
Ph2 = 0.927 atm
Now we will add this information to our Ideal Gas law formula:
PV = nRT
0.927 * 0.081 = n * 0.082 * 313
0.075 = 25.66n
n = 0.003 moles
H2 = 2.0158g/mol
0.003 moles of H2 = 6.0*10^-3
The pH of a solution prepared by mixing 40.00 mL of 0.10 M NH3 with 50.00 mL of 0.10 M NH4Cl and 30mL of 0.05 M H2SO4 is 5.17. Assume that the volume of the solutions are additive . What would be the Ka for NH4
Answers
Answer:
Following are the answer to this question:
Explanation:
The value of pH solution is =5.17 So, the p^{OH}:
\(p^{OH}\)=14-56.17
=8.823
The volume of the \(NH_{3}\) = 40.00 ml
convert into the liter= 0.040L
The value of the concentrated \(NH_{3}\) =0.10 M
The volume of the \(NH_{4}Cl\)= 50.00 ml
convert into the liter= 0.050L
The value of concentrated \(NH_{4}Cl\)= 0.10 M
The volume of the \(H_{2}So_{4}\)= 30 ml
convert into the liter= 0.030L
The value of concentrated \(H_2So_4\)=0.05 M
Calculating total volume=(0.40+0.050+0.030)
=0.120 L
calculating the new concentrated value of \(NH_3\) = \(\frac{0.10\times 0.040}{0.120}= 0.33 \ M\)
calculating the new concentrated value of \(NH_4Cl\)= \(\frac{0.050\times 0.10}{0.120}= 0.04166 \ M\)calculating the new concentrated value of \(H_2So_4= \frac{0.030\times 0.05}{0.120}= 0.0125 \ M\) when 1 mol \(H_2So_4\) produced 2 mols \(H^{+}\) so, 0.0125 in \(H_2So_4\)produced:
\(=4 \times (2 \times 0.0125) \ mol H^{+}\\\\= 0.025 mol H^{+}\)
create the ICE table:
\(NH_3 \ \ \ \ \ \ \ \ + H^{+} \ \ \ \ \ \ \longrightarrow NH_4^{+}\)
I (m) 0.033(m) 0.025 0.04166
C -0.025 -0.025 + 0.025
E 8.3\times 10^{-3} 0 0.0667
now calculating pH:
when ph= 8.83:
\(P^{H}= p^{kb}|+ \log\frac{[NH_4^{+}]}{[NH_3]}\\\\8.83=p^{kb}+\log\frac{0.0667}{8.3 \times 10^{-3}}\\\\p^{kb}=8.83-0.9069\\\\ \ \ \ =7.7231 \\\\\ The P^{kb} \ for \ NH_3 \ is =7.7231\\\\\ The P^{kb} \ for N^{+}H_4=14-7.7231\\\\\ \ \ \ \ \ =6.2769\)
Please please !!! help me I’ve been doing this for 2 hours
How many molecules of NaCl are in 0.61 moles of NaCl?
1.8 x 1023
2.5 x 1023
3.7 x 1023
4.5 x 1023
Answers
Answer:
3.7 x 10^23
is the right answer
Describe three factors that could limit the growth of the prairie dog population
Answers
Answer:
Changes in environment, food source changes and disease?
Explanation:
I dont know what the answer choices are
Answer:
changes in the environment
Explanation:
like digging big holes.
Hope this helps!
How classrooms have become less artificial? Two facts
Answers
Classrooms have grown less artificial by deliberately arranging social activities and games that promote healthy interactions. Students can discuss critical subjects with you in a private setting during class time.
How are classrooms different today?Despite the fact that it's the freshest advancement to schools, remote learning might be trying for the two understudies and teachers. Above projectors and blackboards, previously typical in study halls, have gone in a couple of brief many years, while tablets and workstations have supplanted them as fundamental devices.In a customary training, kids find out about religion, customs, customs, and ceremonies. The students are taught about science, innovation, language capacities, maths, and so forth in the ongoing school system.Innovation based stages like wikis and find out about Docs permit understudies to cooperate on bunch tasks. As innovation offers new techniques for contemplating, imparting, and collaborating, the walls of the homerooms are at this point not an obstruction.Learn more about Modern classroom refer to :
https://brainly.com/question/24204492
#SPJ1
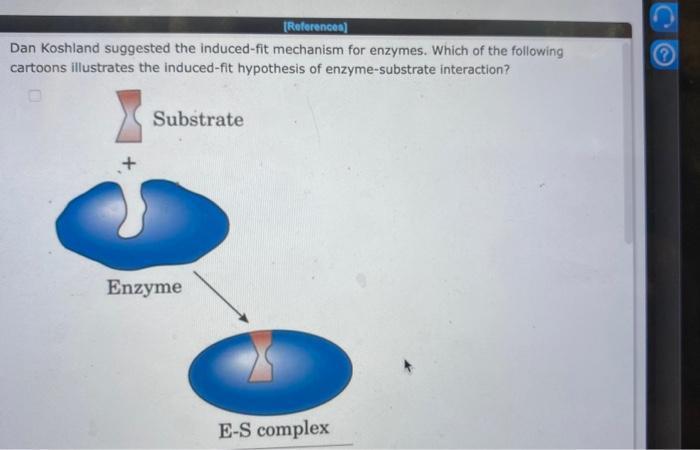
g dan koshland suggested the induced-fit mechanism for enzymes. which of the following cartoons illustrates the induced-fit hypothesis of enzyme-substrate interaction?
Answers
The induced-fit hypothesis of enzyme-substrate interaction illustrates that there is a conformational change in the active site of the enzyme when a substrate binds to it.
According to the induced-fit model, a substrate attaches to an active site, and the two of them slightly alter their shapes to form the perfect fit for catalysis. By bringing substrates together in the best possible orientation, enzymes encourage chemical reactions by establishing the perfect chemistry for the reaction to take place. According to the induced fit hypothesis, an enzyme's active site is not architecturally ideal for substrate binding while it is in the unbound state, or when it is not binding to a substrate. Refer to the image attached for the cartoon illustration.
This induced fit theory extends the lock-and-key idea proposed 100 years ago. In addition to explaining regulatory and cooperative effects, the new theory put out by D. E. Koshland, Jr. in 1958 also provides some new specificity notions. This theory states that an enzyme's unbound active region is not structurally suitable for substrate binding (i.e., not bound to the substrate).
To know more about induced fit theory, refer:
https://brainly.com/question/27548378
#SPJ4
Select the structure that corresponds
to the molecule name:
aniline
B.
A.
-NH₂
C. both
-NH₂
Enter

Answers
Answer:
B- \(C_{6} H_{5} NH_{2}\)Explanation:
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine.


Chemical formula for Aluminum Oxide
Answers
Answer: Al₂O₃
Explanation:
How many atoms of Cr are in 24.95g of chromium

Answers
Atoms of Cr are in 24.95g of chromium is calculated as 2.89 *10^23. The molar mass is the mass in grams of one mole of material.
What is molar mass?In chemistry, molar mass of chemical compound is defined as mass of a sample of that compound divided by the amount of substance which is number of moles in that sample, measured in moles. The molar mass is a bulk and not a molecular property of a substance.
As we know, molar mass of chromium is 52.00 g mol^-1
Avogadro's number is 6.022 *10^23
Number of atoms= (24.95/52) * 6.022 *10^23
So, number of atoms = 2.89 *10^23.
To know more about molar mass, refer
https://brainly.com/question/837939
#SPJ1
The value of the Euro was recently $1.35 U.S. and the price of 1.00 liter of gasoline in France is 1.42 Euro. How many gallons of gas can you buy in France for $1.00?
Answers
List and explain each of the signs of a chemical change.
Answers
- precipitate(solid)forms: becomes cloudy or see sediment settle to the bottom
- release / absorption of energy: change in temperature or gives off light
- color change
mass of 1×10^25 molecules of water
Answers
Answer:
1.E25 it is the answer the answer to mass of 1×10^25 molecules of water
Explanation:
this is just EXPLINATION find your answer using this
first divide the number of molecules by Avogadro's number 6.022*10^25
you will
l get no. of Moles of water
multiply the no. of Moles with mass of 1 Mole of water 18g per mole
if get answer you comment
you should try on your own you will understand better
The energy we get from the sun is called THERMAL ENERGY and the energy from the Earth is called SOLAR RADIATION.
True
or
False
Answers
The solar radiation that reaches the Earth's surface without being diffused is called direct beam solar radiation. The sum of the diffuse and direct solar radiation is called global solar radiation. Atmospheric conditions can reduce direct beam radiation by 10% on clear, dry days and by 100% during thick, cloudy days.
PLS HELP THE QUESTION IS ON THE PICTURE

Answers
Concepts used:
1 mole of an element or a compound has 6.022 * 10²³ formula units
So, we can say that: Number of formula units = number of moles * 6.022*10²³
number of moles of an element or a compound = given mass/molar mass
__________________________________________________________
003 - Number of CaH₂ formula units in 6.065 grams
Number of Moles:
We know that the molar mass of CaH₂ is 42 grams/mol
Number of Moles of CaH₂ = given mass/molar mass
Number of moles = 6.065 / 42
Number of moles = 0.143 moles
Number of Formula units:
Number of formula units = number of moles * 6.022*10²³
= 0.143 * 6.022 * 10²³
= 0.86 * 10²³ formula units
__________________________________________________________
004 - Mass of 6.34 * 10²⁴ formula units of NaBF₄
Number of Moles:
We mentioned this formula before:
Number of formula units = number of moles * 6.022*10²³
Solving it for number of moles, we get:
Number of moles = Number of Formula units / 6.022* 10²³
replacing the variable
Number of moles = 6.34 * 10²⁴ / 6.022*10²³
Number of moles= 10.5 moles
Mass of 10.5 moles of NaBF₄:
Molar mass of NaBF₄ = 38 grams/mol
Mass of 10.5 moles = 10.5 * molar mass
Mass of 10.5 moles = 10.5 * 38
Mass = 399 grams
__________________________________________________________
005 - Number of moles in 9.78 * 10²¹ formula units of CeI₃
Number of Moles:
We have the formula:
Number of moles = Number of Formula units / 6.022* 10²³
replacing the variables
Number of Moles = 9.78 * 10²¹ / 6.022*10²³
Number of Moles = 1.6 / 10²
Number of Moles = 1.6 * 10⁻² moles OR 0.016 moles
the ATOMIC WEIGHT OF ALUMINUM (AL) is 26.98. It has 14 neutrons. Aluminum has ?
a. an atomic mass of 13
b. an atomic number 13
c. 26 electrons
d. 26 protons
I believe b. an atomic number 13. is the answer.
Answers
The atomic weight of Aluminum (AL) is 26.98. It has 14 neutrons. Aluminum has an atomic number 13. Therefore, option B is correct.
What is atomic number?The atomic number of an element represents the number of protons in the nucleus of its atoms. Since the atomic number of an element is unique and determines its position in the periodic table, aluminum has an atomic number of 13.
The number of electrons in a neutral atom is equal to the number of protons, which in this case is 13. Therefore, aluminum has 13 electrons.
The atomic weight of aluminum (Al) is 26.98, which represents the weighted average mass of all the naturally occurring isotopes of aluminum, taking into account their relative abundances. The number of neutrons in an atom of aluminum is given as 14.
Thus, the correct option is B.
To learn more about the atomic number, follow the link:
https://brainly.com/question/16858932
#SPJ3
A neutral atom of Sodium (Na) has an atomic number of 11 and an atomic mass of 23. Therefore, Na has _________________________ protons.
Answers
Answer:
11
Explanation:
Atomic number is the number of PROTONS an element has .
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
Consider the reaction of solid aluminum iodide and potassium metal to form solid potassium iodide and aluminum metal.
Write the balanced chemical equation for the reaction.
Answers
The balanced chemical equation is AlI₃(s) + 3 K(s) → 3 KI(s) + Al(s). The number of moles of aluminium will be 2.437moles.
What is chemical equation ?Chemical equations are symbols and chemical formulas that depict a chemical reaction symbolically.The reactive species and end product are described by the chemical equation. The mole ratio or molecular ratio of the constituent components or compounds in the reaction is revealed by the coefficient of the reacting species and the products that are produced.Reactants are the substance(s) in a chemical equation to the left of the arrow. A component that is present at the outset of a chemical reaction is known as a reactant. Products are the substance(s) to the right of the arrow. A substance that remains after a chemical reaction is complete is known as a product.To learn more about chemical equation refer :
https://brainly.com/question/26227625
#SPJ1
