Answers
Answer:
So sorry if I was wrong but I think it's B. Because from the source states,
https://socratic.org/questions/which-group-on-the-periodic-table-is-the-least-reactive-why
"The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium, neon, radon or the transition elements."
Related Questions
A scientist did a test to compare two substances: substance Q and substance R. At room temperature, both substances are liquid. When the scientist transferred the same amount of energy out of both substances, only one substance changed phase while the other did not. Which substance changed phase, and how did it change?
Responses
a
Substance Q changed phase because the attraction of the molecules was able to overcome their slower movement. Its molecules now move in place.
Substance Q changed phase because the attraction of the molecules was able to overcome their slower movement. Its molecules now move in place.
b
Substance Q changed phase because the strong attraction between molecules made their movement slower. Its molecules now move in place.
Substance Q changed phase because the strong attraction between molecules made their movement slower. Its molecules now move in place.
c
Substance R changed phase because the weak attraction between molecules let them move faster. Its molecules now move around each other.
Substance R changed phase because the weak attraction between molecules let them move faster. Its molecules now move around each other.
d
Substance R changed phase because the attraction was able to overcome the slower molecules. Its molecules now move away from each other.
Substance R changed phase because the attraction was able to overcome the slower molecules. Its molecules now move away from each other.
Answers
Substance R changed phase because the weak attraction between molecules let them move faster. Option C
What is phase change?We know that a change of phase has to do with the movement of a material from a given state of matter to the other. Net us note that when there is a change of state, we are going to notice that the force of attraction in the molecule would also change.
Since we know that the substance that we have are liquid at room temperature, we know that the supply of some energy may cause to be bale to change its phase.
Learn more about phase change:https://brainly.com/question/12390797
#SPJ1
Part EAscorbic acid, or vitamin C (Ascorbic molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. A healthy adults daily requirement of vitamin C is 70-90 mg. A sweet lime contains 2.88 x 10^4 mol of ascorbic acid.To determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.88 x 10^4 mol of ascorbic acid.Express the mass in grams to three significant figures.You did not open hints for this part
Answers
Explanation
Given:
Molar mass of ascorbic acid, M = 176 g/mol
Moles of ascorbic acid in sweet lime = 2.88 x 10⁴ mol
what to find:
The mass of ascorbic acid in 2.88 x 10⁴ mol of ascorbic acid.
Step-by-step solution:
The relationship between mole, n, reacting mass, m, and molar mass, M, is given by
\(\text{mole (n) }=\frac{Reac\text{ting mass (}m)}{Molar\text{ mass (}M)}\)So, substitute the given moles and molar mass of ascorbic acid into the formula above to get the mass of ascorbic acid:
\(undefined\)what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
What is the mass of a single bromine molecule, Br2?
Answers
The mass of a single bromine molecule, Br₂ is 2.654 x 10⁻²² g.
Bromine is a diatomic element. It has a symbol Br and the atomic number is 35. Bromine is a naturally occurring element which is liquid at room temperature.
Molar mass of Br₂ is 159.808 g/mol. The molar mass of bromine is 79.904 g/mol. In Br₂, there are two bromine atoms.
To calculate the mass of a single bromine molecule -
In 1 mole of Br₂, there is Avogadro's number of molecules.
Avogadro's number = 6.022 x 10²³
Therefore, 1 mole = 6.022 x 10²³
1 mole of bromine has mass of 79.9 g/mol
So, it gives us -
1/6.022 x 10²³ = 1.66 x 10²³/79.9 = 2.654 x 10⁻²²g
For the mass of a single bromine molecule, Br₂ = 2.654 x 10⁻²² g
To learn more about bromine,
brainly.com/question/9080164
#SPJ1
Exploring
A change that produces matter with a different composition is
called a
a chemical change
b radical change
c physical change
d transformational change
Check
Answers
Answer:
A chemical change
Explanation:
A chemical change is a change that produces matter with a different composition than the original matter
Copper metal reacts with a solution of silver nitrate, AgNO3, to produce copper (II) nitrate and silver metal. In carrying out this reaction, a piece of copper wire was immersed in a solution of silver nitrate until the reaction stopped. The original mass of the copper wire was 2.36 grams. After the reaction stopped, the mass of the wire was 1.03 grams. What mass of silver was produced?
Answers
Despite the long text, this is another stoichiometry question, I will follow the same step by step I have explained in our previous session
1. Balancing the equation:
Cu + 2 AgNO3 -> Cu(NO3)2 + 2 Ag, now the reaction is properly balanced
2. If we had 2.36 grams of Cu and only 1.03 grams are left, this means that we had copper in excess, and only 1.33 grams actually reacted, using the molar mass of Cu, 63.55 g/mol, we can find the number of moles:
63.55g = 1 mol
1.33g = x moles
x = 0.02 moles of Cu
3. Finding the molar ratio, which we can find by looking at the reaction, between Cu and Ag, the molar ratio is 1:2, which means 1 mol of Copper to produce 2 moles of silver, therefore if we have 0.02 moles of Cu, we will end up with 2 times this value, which is 0.04 moles of Ag
4. Now we have the number of moles of Ag, and its molar mass is, 107.87g/mol
107.87g = 1 mol
x grams = 0.04 moles
x = 4.31 grams of Silver will be produced from 1.33 grams of Copper
If data from a clinical trial contains personal information about a study participant, which process should be used to separate identifying information from health information?
A. De-identification
B. Informed consent
C. ALCOA
D. Good documentation practices
Answers
If data from a clinical trial contains personal information about a study participant, De-identification process should be used to separate identifying information from health information. Thus, Option (A) is correct
What is De-identification process ?De-identification is a process of detecting identifiers (e.g., personal names and social security numbers) that directly or indirectly point to a person (or entity) and deleting those identifiers from the data.
De-identification and anonymization are strategies that are used to remove patient identifiers in electronic health record (EHR) data.
Hence, De-identification process should be used to separate identifying information from health information. Thus, Option (A) is correct.
learn More about health record here ;
https://brainly.com/question/11156017
#SPJ1
Which of the following societies would have the lowest environmental impact?
A populous, highly industrialized society with high levels of consumption.
A less populated, highly industrialized society with moderate consumption levels.
A small population that farms using hand tools, has no modern technology, and grows their own food.
A large population with moderate industrialization and consumption levels.
Answers
The society with the least negative effects on the environment is probably the one with a small population, traditional farming methods, no access to contemporary technology, and self-sufficient food production.
This is due to the fact that their way of living is less dependent on modern infrastructure and technology, both of which have a negative impact on the environment. Additionally, their agricultural methods are probably more environmentally friendly and sustainable.
The environmental effects of the other societies on the list would all be greater. Because of the use of fossil fuels and the production of products that require a lot of resources, a big, industrialized society with high levels of consumption would have a significant carbon footprint.
It would still take a lot of resources to maintain its infrastructure and create products in a less populous, highly industrialized society with moderate consumption levels, which would have a negative effect on the environment.
Given that the size of the population alone would necessitate significant resource consumption and infrastructure development, a big population with moderate industrialization and consumption levels would also have a big effect on the environment.
learn more about the population here
https://brainly.com/question/29885712
#SPJ1
please help i am losing motivation and very depressed please look at photo and answer.

Answers
Answer:
Solid turns to liquid.
Explanation:
When you freeze water, it turns into ice. When melted it's still water. No chemical changes were made to the piece of ice melted. The only change was ice (solid) to water (liquid)
Conservation of Matter - Matter can change forms through physical and chemical changes, but no matter what, matter is conserved. The same amount of matter exists before and after the change.
Hope this helps! Please mark brainliest!
How many electrons are in the 6p subshell of Rn?
Answers
In the electrical arrangement, the 6p subshell of the radon element contains 6 electrons.
What is electronic configuration of radon?Radon is the 86th element in the periodic table, with the symbol 'Rn'. Radon is a type of noble gas. Radon contains a total of eighty-six electrons. These electrons are grouped in accordance with the laws of several orbits.The p-orbital can hold up to six electrons. As a result, the following six electrons enter the 2p orbital. The second orbit is now completely filled. As a result, the remaining electrons will go into the third orbit.As a result, the entire electron configuration of radon is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 4f^14 5s^2 5p^6 5d^10 6s^2 6p^6.For more information on electronic configuration kindly visit to
https://brainly.com/question/29757010
#SPJ1
I need a lil help here plzzzzzzzz

Answers
Answer:
The southern hemisphere is currently experiencing "Summer"
Explanation:
The Southern hemisphere is experiencing summer because it is closer to the sun than the Northern Hemisphere and also, direct light is more hotter than indirect light.
So, first, let's try an EXPERIMENT!
Take a VERY bright flashlight or a very small laser that can't harm you or just burn a paper in 1 second.
Then, take a piece of paper, or maybe just your skin, (like your hand) and place it under the light or laser, (for the laser, you should probably take the paper), and place the light source right above your skin, then, after some time, when it gets hot, measure the temperature. (around like 30 seconds later),
Note down the temperature...
Now, do the same thing, accept, do it in a very weird angle, face it towards your paper or hand, but, do it in an angle. More than like 30 degrees. Then, after some time, note down the temperature.
You probably, noticed, that the direct light (the one with the straight light facing your paper or skin, was hotter). Look at the image below, and you can understand better.
So, the same thing applies to the earth. The southern hemisphere in this picture is facing more directly to the sun than the northern hemisphere. That is why your answer is
SUMMERThank you! Please mark me Brianliest!
Remember to have fun, and a nice day!

Answer: summer
Explanation:
How would you prepare 0.400L of 0.155M Sr(OH)2 from solid Sr(OH)2?
Answers
Initially, we will consider only solutions of a solid in ... (d) Calculate the volume (in mL) of 0.1065 M Sr(OH)2 required to titrate 25.00 mL of 0.2465 M.
:
Explanation:
CAN SOMEONE HELP WITH THIS QUESTION?

Answers
The minimum mass of hydrochloric acid that could be left over by the chemical reaction is 18.23 g.
What is moles?Moles in chemistry is a unit of measurement for the amount of a substance. It is equal to the number of atoms or molecules in a given mass of a substance, and is represented by the symbol ‘mol’. The molar mass of a substance is the mass of one mole of that substance, expressed in grams. It is important for calculating the amount of energy or reactants needed for a reaction. Moles can also be used to measure the concentration of a solution.
The minimum mass of hydrochloric acid that could be left over by this chemical reaction can be calculated using the following equation:
Moles of HCl = Mass HCl / Molar Mass HCl
Moles of NaOH = Mass NaOH / Molar Mass NaOH
Moles of HCl must equal moles of NaOH in the reaction, so:
Moles HCl = Moles NaOH
Mass HCl / Molar Mass HCl = Mass NaOH / Molar Mass NaOH
Rearranging for mass HCl:
Mass HCl = Molar Mass HCl x Mass NaOH / Molar Mass NaOH
Given:
Mass HCl = 28.8 g
Molar Mass HCl = 36.46 g/mol
Mass NaOH = 20.0 g
Molar Mass NaOH = 40.0 g/mol
Substituting values:
Mass HCl = 36.46 g/mol x 20.0 g / 40.0 g/mol
Mass HCl = 18.23 g
Therefore, the minimum mass of hydrochloric acid that could be left over by the chemical reaction is 18.23 g.
To know more about moles click-
https://brainly.com/question/15356425
#SPJ1
For the reaction:
H₂O(1)→ H₂O(g)
ΔΗ = 44 kJ
How much heat is required when 9.0 g of H₂O is used?
Answers
1mol H_2O requires 44kJ heat
Moles of water
Given mass/Molar mass9/181/20.5molHeat required
0.5(44)22kJAs per the reaction given
Moles of H_2O=1
∆H=44kJMoles of water in 9g
Given mass/Molar mass9g/18g/mol0.5mol∆H
44(0.5)22KJWhat solution does ?

Answers
Water can be separated into hydrogen and oxygen gas via electrolysis in the following equation. 2H20 -> 2H2 +O2
What type of reaction is this.

Answers
Answer:
D: Decomposition Reaction
Explanation:
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.
The general form of a decomposition reaction is: AB -> A+B.
You just split the nonmetals into a molecule depending on the element.
But they are rules though when doing this reaction. If a element is a diatomic molecule, then you would write a 2 in the subscript.
Diatomic molecules contain two atoms that are chemically bonded
The 7 diatomic elements are: H: Hydrogen H2N: Nitrogen N2F: Fluorine F2O: Oxygen O2I: Iodine I2C: Carbon C2B: Bromine B2Now let us go back to this problem: 2H2O -> 2H2 + O2
So water or 2H2O split into 2H2 and O2 because hydrogen and oxygen are diatomic elements.
That is how you write the product of a decomposition reaction.
Hope it helped!
The United States experienced a decrease in the real GDP, high inflation, and a
rising unemployment rate. The United States
was in the middle of an economic boom
appeared to be entering a recession
was in an economic slump
was in a stagnant economic period
Answers
The United States experienced a decrease in the real GDP, high inflation, and arising unemployment rate.
The United States appeared to be entering a recession.A recession is a decline in economic activity, characterized by declining GDP, high unemployment rates, and increased unemployment benefits. Economic analysts and the media commonly use a two-quarter consecutive decline in real GDP as a definition of a recession.
The United States is considered to have entered a recession in the 1970s, which was characterized by an energy crisis, inflation, and recession. However, by the end of the decade, the economy had improved, and it entered into the 1980s with a strong economic performance.
The 1970s were a period of high inflation, low growth, and an oil crisis, which had a significant impact on the United States economy. Therefore, it can be concluded that The United States was in the middle of an economic boom before the 1970s recession and entered a recession in the 1970s due to a decrease in the real GDP, high inflation, and arising unemployment rate.
For more such questions on inflation visit;
https://brainly.com/question/28061405
#SPJ8
What mass (in kg) does 4.64 moles of Copper have?
Answers
0.294kg is mass (in kg) that 4.64 moles of Copper have.
What does a mole mean?The unit of material quantity in the International System of Units is the mole (SI). A substance's quantity determines how many elementary entities of that substance are contained in an object or sample. The number of elementary entities in a mole is precisely 6.02214076 × 10²³.
The amount of a substance in a mole is determined by the mass of a mole of that substance divided by the mass of the substance given in a chemical reaction. The term is also used to express concentration measurements like mole per litre and molecular weight.
m= n × MM
m is mass in grams; n is moles & MM is molecular mass
The molecular mass of copper is 63.546.
We now have all the required information to perform the calculation
m= 4.64 × 63.546
m= 294 g
= 0.294kg
To learn more about molecular weight use link below:
https://brainly.com/question/14596840
#SPJ1
It takes 4 pounds of steel to make a small robot. You have 48 ounces. Do you have enough? If not what do you need?
Answers
No, 48 ounces are not enough. For making a small robot we need 64 ounces which is equal to 4 pounds.
What is pound and ounces?Pound is a unit for measuring weight. 16 ounces makes one pound.
Ounce is also a unit for measuring weight. 16 ounces is equal to 1 pound
So, for making one small robot we need 4 pounds.
1 pound = 16 ounces
4 pounds = 64 ounces
But, we have 48 ounces
We need more = 64 - 48 = 16 ounces or 1 pound
No, 48 ounces are not enough. For making a small robot we need 64 ounces which is equal to 4 pounds.
To know more about pounds, check out:
https://brainly.com/question/22599208
#SPJ1
Are sperm and egg cells exact copies of the plant cell
Answers
Answer:
No
Explanation:
thats scientifically impossible
Scientists found the remains of dinosaur fossils in a remote section in the west, located
within ten miles of a meteor crash site. Which statement can be made about the
dinosaur and the meteor?
O a. The dinosaur could have died from a flood.
O b. The dinosaur and the meteor are not related.
O c. The dinosaur could have died from freezing to death.
O d. The dinosaur could have died due to an meteor crash.
Answers
Answer:
D The dinosaur could have died due to an meteor crash.
Explanation:
Answer:
D
Explanation:
The dinosaurs fossils were found ten miles away from the meteor crash site. Correct me if I'm wrong
For the molecule, GeO2, determine its Lewis structure, bonding pairs/lone pairs, electron geometry, its shape, and whether or not the molecule is polar.
Answers
The correct answers to the problem given above about germanium IV oxide are as follows:
a. The Lewis structure of the molecule; GeO₂ simply is O = Ge = Ob. The bonding pairs/lone pairs of the molecule GeO₂ are two bond electron pairs and four electrons respectively.c. The electron geometry of GeO₂ is linear geometry d. The shape of germanium IV oxide is α-quartz type hexagonal structuree. Germanium oxide is a polar molecule.What is meant by GeO₂GeO₂ is a chemical compound also known as germanium oxide, germanium dioxide or germanium IV oxide. It is a polar covalent which is a soluble substance to some extent.
In conclusion, it can be deduced from the explanation given above that germanium IV oxide is a chemical compound.
The complete lewis structure of germanium IV oxide is attached.
Complete question:
Answer the following questions about germanium IV oxide, GeO₂
a. Determine its lewis structure.
b. Its bonding pairs/lone pairs
c. What is its electron geometry (GeO₂)?
d. Its shape (GeO₂)?
e. Is germanium IV oxide a polar or non-polar molecule?
Read more on lewis structure:
https://brainly.com/question/11554350
#SPJ1
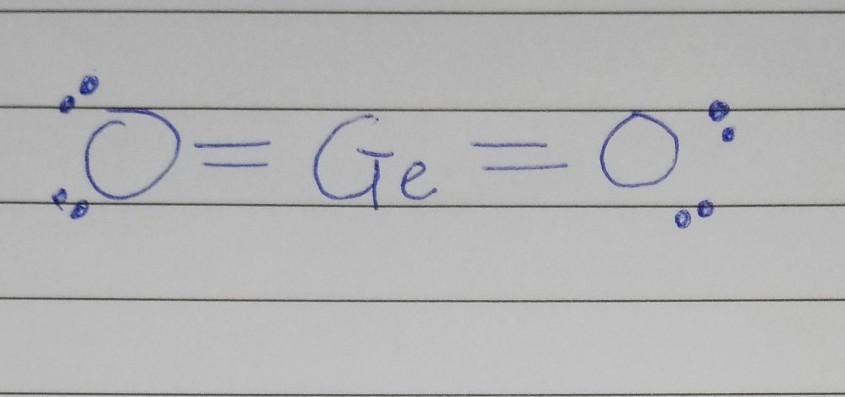
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
A hospital saline solution is analyzed to confirm its concentration. A 50.0 mL sample with a mass of 50.320 g is evaporated to dryness. If the solid sodium chloride residue has a
mass of 0.669 g. what is the mass percent and molar concentration of the saline solution?
Answers
The mass percent of the sodium chloride in the saline solution is approximately 1.33%. The molar concentration of the saline solution is approximately 0.229 M.
To determine the mass percent and molar concentration of the saline solution, we need to analyze the mass of the sodium chloride residue and the initial mass of the sample.
Mass percent:
The mass percent is calculated by dividing the mass of the sodium chloride residue by the initial mass of the sample and then multiplying by 100%.
Mass percent = (Mass of NaCl / Initial mass of sample) × 100%
Mass of NaCl = 0.669 g
Initial mass of sample = 50.320 g
Mass percent = (0.669 g / 50.320 g) × 100% ≈ 1.33%
The mass percent of the sodium chloride in the saline solution is approximately 1.33%.
Molar concentration:
To calculate the molar concentration of the saline solution, we need to determine the number of moles of sodium chloride and the volume of the solution.
Moles of NaCl = Mass of NaCl / Molar mass of NaCl
The molar mass of NaCl is 58.44 g/mol.
Moles of NaCl = 0.669 g / 58.44 g/mol ≈ 0.01144 mol
Since the volume of the sample is given as 50.0 mL, we need to convert it to liters.
Volume of solution = 50.0 mL = 50.0 mL × (1 L / 1000 mL) = 0.0500 L
Now we can calculate the molar concentration (Molarity) using the formula:
Molarity (M) = Moles of solute / Volume of solution (in liters)
Molarity = 0.01144 mol / 0.0500 L ≈ 0.229 M
The molar concentration of the saline solution is approximately 0.229 M.
for more such questions on concentration
https://brainly.com/question/17206790
#SPJ11
A buffer solution is prepared by dissolving 0.2 mol of CH3NH2 (KB=3.7×10−4) and 0.1 mol of CH3NH3Cl in enough water to make 1.00 L of solution. Determine the pH of the buffer
Answers
The pH of acid is between 0-7 on pH scale while for base pH range is from 7-14. Therefore, the pH of buffer is 10.5. pH is a unitless quantity.
What is pH?pH is a measurement of amount of hydronium ion H₃O⁺ in a given sample. More the value of hydronium ion concentration, more will be the solution acidic.
On subtracting pH from 14, we get pOH which measures the concentration of hydroxide ion in a given solution. pH depend on the temperature. At room temperature pH scale is between 0 to 14. pH of neutral solution is 7.
CH\(_3\)NH\(_2\) + H\(_2\)O(aq) \(\rightarrow\) CH\(_3\)NH\(_3\) ⁺(aq) + OH⁻(aq)
Kb = [CH\(_3\)NH\(_3\) ⁺] x[OH⁻] =3.7x 10⁻⁴
[CH\(_3\)NH\(_2\)]= 0.2 / 1.00 =0.2M
Kb = (0.2M) x [OH⁻] = 3.7x 10⁻⁴
[OH-] = 3.15 x 10⁻⁴
pOH=-log[OH-]
= -log[3.15 x 10⁻⁴]
pOH=3.5
pH+pOH=14
pH = 10.5
Therefore, the pH of buffer is 10.5.
To learn more about pH, here:
https://brainly.com/question/27945512
#SPJ1
How many water molecule is lost when each molecules of this hydrate is heated Na2SO4•10H2O ?
Answers
When each molecule of Na₂SO4•10H₂O is heated, it loses 10 water molecules.
How many water molecule is lost in Na2SO4•10H2O ?When Na₂SO₄•10H₂O is heated, it loses water molecules to become anhydrous Na₂SO₄.
The number of water molecules lost can be calculated as follows:
Each molecule of Na₂SO₄•10H₂O contains 10 water molecules, so when it is heated, it loses all of these water molecules.
Therefore, when each molecule of Na₂SO4•10H₂O is heated, it loses 10 water molecules.
Learn more about water molecules here: https://brainly.com/question/30945925
#SPJ1
PLEASE HELPPP ME
Use the following balanced chemcial equation to answer the question below.
CaBr2 + Na2SO4 --> CaSO4 + 2NaBr
1. How many moles of sodium bromide can be made from 5 moles of calcium bromide
2. How many grams of calcium sulfate will be made from 2.00 moles of calcium bromide
3. How many grams of calcium bromide will be needed to make 845 grams of calcium sulfate
Answers
Answer:
1) (5 moles of calcium bromide) x 2 / 1 = 10 moles
2) (2.00 moles of calcium bromide) = 2.00 moles of calcium sulfate
so (2.00) x (136.1 g/mol) = 272.2 g
3) (845 grams of calcium sulfate) / 136.14 g/mol = 6.21 moles
6.21 moles of calcium sulfate = 6.21 moles of calcium bromide
(6.21 moles of calcium bromide) x 199.89 g/mol = 1241.32 g
please help mee!!!!
Answers
Which is one way that spring tides are different from regular tides?
Answers
Answer:
high tides are a little higher and low tides are a little lower than average
Explanation:
A spring tide is the highest tide (when the greatest difference between the high and low tides). This happens during the new and full moon.
Answer: It's worth noting that low tides can sometimes be lower than usual, which is referred to as spring tides. Despite its name, this phenomenon isn't related to spring and has a different historical origin.
How does Gas Trapping bottles Monitor Volcanos?
( put in your own words don’t look up on internet )
I will give brainlst If you answer correctly.
Answers
Answer: the pressure releases gas. The two most abundant gases are sulfur dioxide and carbon dioxide, and if levels of these gases increase,
Explanation:
