Plants need to take in gases and release gases during their
everyday life processes. What part allows plants to do this?
Answers
Answer:
The Stomata
Explanation:
The Stomata is found in the leaves of plants and controls their gas exchange with the environment around them.
Related Questions
Distinguish between an acidic and a basic oxide.
(Select all that apply.)
1. In a basic oxide, element forms ionic bonds with oxygen.
2. A basic oxide is an oxide that reacts with bases.
3. A basic oxide is an oxide that reacts with acids.
4. In a basic oxide, element forms covalent bonds with oxygen.
5. An acidic oxide is an oxide that reacts with acids.
6. In an acidic oxide, element forms ionic bonds with oxygen.
7. In an acidic oxide, element forms covalent bonds with oxygen.
8. An acidic oxide is an oxide that reacts with bases.
Answers
Answer:
See below ~
Explanation:
Basic oxides
In a basic oxide, element forms ionic bonds with oxygenA basic oxide is an oxide that reacts with basesAcidic oxides
An acidic oxide is an oxide that reacts with acidsIn an acidic oxide, element forms covalent bonds with oxygenHey besties can y'all help me out
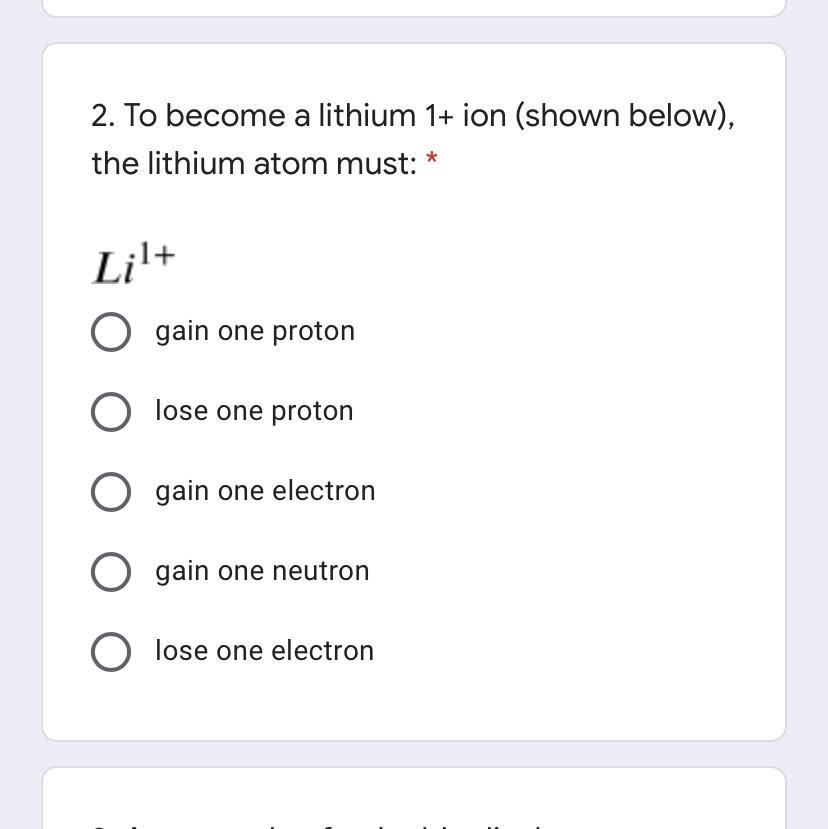
Answers
you need to lose one electron
Mix 5.40 g of Al with sufficient of Cl2. What mass of AlCl3 can form
Answers
A water solution containing an unknown quantity of a
nonelectrolyte solute is found to have a freezing point of
–0.23°C. What is the molal concentration of the solution?
Answers
Answer:
The molal concentration of the solution is approximately 0.124 molal
Explanation:
The given parameters are;
The reduced freezing point of the water = -0.23°C
For freezing point depression of a solution, we have;
\(\Delta T_f\) = \(K_f\)· b·i
Where;
\(\Delta T_f\) = The freezing point depression
\(K_f\) = Cyroscopic constant (The freezing-point depression constant) = 1.86° Cm⁻¹ for water
b = The molality of the solution
i = van't Hoff factor = 1
Therefore;
\(\Delta T_f\) = \(T^{\circ}_f - T_f\)
Where;
\(T^{\circ}_f\) = The freezing point of pure water = 0°C
\(T_f\) = The freezing point of the solution = -0.23°C
∴ \(\Delta T_f\) = \(T^{\circ}_f - T_f\) = 0°C - (-0.23°C) = 0.23 °C
From, \(\Delta T_f\) = \(K_f\)· b·i, we have;
0.23°C = 1.86°C/m × b × 1
∴ b = (0.23°C/1.86°C/m) ≈ 0.124 molal
Therefore, the molal concentration of the solution, b ≈ 0.124 molal.
H2O trong hóa học là gì? Ngoài đời là gì?
Answers
Answer:
Ký hiệu hóa học (xem thêm ký hiệu) cho nước. Mỗi phân tử nước chứa hai nguyên tử hydro (H) liên kết với một nguyên tử oxy (O).
The chemical symbol (see also symbol) for water. Each molecule of water contains two atoms of hydrogen (H) joined to a single atom of oxygen (O).
nascent hydrogen is more reactive than molecular hydrogen justify your answer
Answers
Nascent hydrogen is more reactive than molecular hydrogen simply because nascent hydrogen is more reactive than molecular hydrogen.
This released energy activates the nascent hydrogen and makes it more energy rich than that of ordinary occurring molecular hydrogen.
What is an element?An element can be defined as a substance which cannot be split into simpler units by an ordinary chemical process.
Below are examples of some chemical elements
HydrogenHeliumLithiumBerryliumBoronCarbonNitrogenOxygenFluorineNeonSodiumMagnesiumSiliconSo therefore, nascent hydrogen is more reactive than molecular hydrogen simply because nascent hydrogen is more reactive than molecular hydrogen.
Learn more about chemical elements:
https://brainly.com/question/7289437
#SPJ1
5. Modern houses have systems to control internal air temperatures in times of very cold or hot weather. In these homes, hot or cool air is pumped into the interior of the house to help keep the temperature comfortable. Based on what you know about how cool and warm air circulate in the atmosphere, what might an engineer need to consider when designing a heating/cooling system for a home in a cooler climate?
How might the design differ for a house found in a warmer climate?
Answers
An engineer need to introduce heat pump systems when designing a heating/cooling system for a home in a cooler or warmer climate.
For all climates, heat pumps provide a more energy-efficient furnace and air conditioner replacement. Heat pumps, like your refrigerator, use power to move heat from a cold space to a warm space, warming the warm space and making the cool space cooler. Therefore, an engineer may introduce heat pump systems while designing a house in any kind of climate.
In the winter, heat pumps transfer heat from the chilly outside to your comfortable home. In the summer, heat pumps transfer heat from your home to the outside.
Heat pumps can effectively supply your home with acceptable temperatures since they transfer heat rather than producing it.
To know more about heat pump systems, refer to the following link:
https://brainly.com/question/3150928
#SPJ1
and non-flammable gases
Noble gases are
that have low chemical

Answers
Answer:
Noble gases are odorless, colorless, and nonflammable gases that have low chemical reactivity.The full valence electron shells of these atoms make noble gases extremely stable.& they are unlikely to form chemical bonds because they have little tendency to gain or loseelectrons.
Which example is Not an example of Chemical Potential energy. The hamburger, The Spring, The battery, the flask
Answers
Answer:
Hamburger
Explanation:
What is vibrational frequency directly proportional to in IR spectroscopy?
Answers
In IR spectroscopy, vibrational frequency is directly proportional to the energy of the vibrational mode, which in turn is proportional to the strength of the chemical bond and the masses of the atoms involved in the bond.
In infrared (IR) spectroscopy, vibrational frequency is directly proportional to the energy of the vibrational mode. Specifically, the vibrational frequency of a molecule is directly proportional to the strength of the chemical bond and the masses of the atoms involved in the bond. As the vibrational frequency increases, the energy of the bond also increases. This is because the bond becomes stiffer and requires more energy to vibrate. Therefore, IR spectroscopy can be used to identify the types of chemical bonds present in a molecule by measuring the vibrational frequencies of the bonds.
Learn more about IR spectroscopy here: brainly.com/question/28543039
#SPJ4
When you mix silver nitrate and hydrochloric acid is it a physical or chemical change?
Answers
Answer:
When you mix silver nitrate and hydrochloric acid it is a chemical
change.
Explanation:
Physical change: You are changing the form of a chemical substance, but not, chemical composition.
- Physical changes are used to separate mixtures into their component compounds, but cannot usually be used to separate compounds into chemical elements or simple compounds.
Chemical Change: Occurs when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances.
- These processes are called chemical reactions and, in general, are not reversible except by further chemical reactions.
When you mix silver nitrate and hydrochloric acid it is a chemical change.
What is physical change?
Physical changes are changes which affecting the form of chemical substances but not it's chemical composition. Physical change are the processes in which we can separate mixture from compound, but can't usually be used to separate compound into chemical elements or simplest compound.
Physical change takes place when mixture are can't separate by chemical changes then we separate by the help of physical change. In general a physical change is reversible using physical means.
Silver is a shiny metal that conducts electricity very well . It can be molded into thin sheets it means it can spread into a like a sheet and these type of properties is known as malleability.
Potassium is dull and brittle it means that it can't be change into sheet when you try to change in sheet it will break and these properties are called as brittlness. And when it is has been dissolved into water, which it does quite easily. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting points and boling points.
Therefore, When you mix silver nitrate and hydrochloric acid it is a chemical change.
Learn more about physical change here:
https://brainly.com/question/17931044
#SPJ2
how do you determine the thermal energy of an object
Answers
Answer:
An object's thermal energy is dependent upon its temperature and mass. The higher the temperature of a given quantity of a substance, more is its thermal energy. Similarly, for the same temperature, higher mass of a substance will contain more thermal energy
4) How many grams are in 4.63 x 102 moles of CC14?
Answers
Answer:
n=mass/molar mass
mass=?,molar mass=12+(35.5)4=154g/mol.n=4.63x10²
mass=4.63x10²x154=7128g
Write the formulae of the acid and base of ZnS.
Answers
ZnS is the chemical formula of the zinc sulphide. Zinc sulphide contain two ions i.e. ( Zn ⁺² ) and ( S ⁻² ) ion present in zinc sulphide.
What is acid ?A Bronsted - Lowry acid or Lewis acid is a molecule or ion that has the ability to donate a proton or establish a covalent bond with an electron pair. Proton donors, also known as Bronsted - Lowry acids, are the first class of acids.
Any hydrogen containing material that has the ability to donate a proton (hydrogen ion) to another chemical is considered an acid. A base is a molecule or ion that can take up an acid's hydrogen ion. Typically, sour tastes help to identify acidic chemicals.
Thus, ZnS is the chemical formula of the zinc sulphide. Zinc sulphide contain two ions i.e. ( Zn ⁺² ) and ( S ⁻² ) ion present in zinc sulphide.
To learn more about acid follow the link below;
https://brainly.com/question/14072179
#SPJ1
What characteristics best describe an asteroid?
It revolves around a planet and is larger than the planet.
It revolves around a planet and is much smaller than the planet.
It revolves around the sun and is much smaller than a planet.
It rotates on its axis and is the center of the solar system.
Answers
Answer:
C
Explanation:
Take my word.
Hope this helped.
Answer:
C: It revolves around the sun and is much smaller than a planet.
Explanation:
An asteroid is a rocky or metallic object that orbits, revolves around, the Sun and is much smaller than a planet. - Trust your Teacher here.
how many grams are in 22.3 mole of Copper (II) Hydroxide?
Answers
Answer:
2180 g Cu(OH)₂
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
22.3 mol Cu(OH)₂
Step 2: Identify Conversions
Molar Mass of Cu - 63.55 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H - 1.01 g/mol
Molar Mass of Cu(OH)₂ - 63.55 + 2(16.00) + 2(1.01) = 97.57 g/mol
Step 3: Convert
\(22.3 \ mol \ Cu(OH)_2(\frac{97.57 \ g \ Cu(OH)_2}{1 \ mol \ Cu(OH)_2} )\) = 2175.81 g Cu(OH)₂
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
2175.81 g Cu(OH)₂ ≈ 2180 g Cu(OH)₂
Consider this reaction:
At a certain temperature it obeys this rate law.
rate
Suppose a vessel containsat a concentration of. Calculate the concentration ofin the vesselseconds later. You may assume no other reaction is important
Answers
The concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B].
We use the initial concentration of A and B and the rate constant of the reaction to find the rates at these concentrations. Using the integrated rate law for a second-order reaction, we find the concentration of A after 30 seconds to be 0.0934 M.
Given reaction obeys the rate law, rate=k[A]²[B].
Here, the initial concentration of A= 0.10 M,
initial concentration of B = 0.05 M, and
rate constant, k = 2.0 × 10⁻⁴ M⁻¹s⁻¹
We have to find the concentration of A, after 30 seconds.
To find the concentration of A, we need to know the rate at 0.10 M and 0.05 M. Therefore, we have to calculate the rates at these concentrations.
rate1 = k[A]²[B]
= (2.0 × 10⁻⁴ M⁻¹s⁻¹)(0.10 M)²(0.05 M)
= 1.0 × 10⁻⁷ M/srate2
= k[A]²[B] = (2.0 × 10⁻⁴ M⁻¹s⁻¹)(0.09 M)²(0.04 M)
= 6.48 × 10⁻⁸ M/s
Using the integrated rate law for a second-order reaction: [A] = [A]₀ - kt where [A]₀ = initial concentration of A, k = rate constant, and t = time in seconds.
We know [A]₀ = 0.10 M and k = 2.0 × 10⁻⁴ M⁻¹s⁻¹.
Substituting the values in the above equation, we get: [A] = [A]₀ - kt= 0.10 M - (2.0 × 10⁻⁴ M⁻¹s⁻¹)(30 s)≈ 0.0934 M
Therefore, the concentration of A in the vessel after 30 seconds is 0.0934 M.
This question requires us to calculate the concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B].
We are given the initial concentration of A and B and the rate constant of the reaction. To find the concentration of A after 30 seconds, we need to calculate the rates at the initial concentrations of A and B.
Using the integrated rate law for a second-order reaction, we can find the concentration of A at any given time. We substitute the given values in the formula and solve for [A]. We get the concentration of A as 0.0934 M after 30 seconds. This calculation is based on the assumption that no other reaction is important.
The concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B]. We use the initial concentration of A and B and the rate constant of the reaction to find the rates at these concentrations. Using the integrated rate law for a second-order reaction, we find the concentration of A after 30 seconds to be 0.0934 M. This calculation assumes that no other reaction is important.
To know more about concentration visit:
brainly.com/question/13872928
#SPJ11
You are required to go to the lab and test the combustion of calcium carbonate. Calcium carbonate is a white mineral. You burned calcium carbonate, CaCO3, to produce carbon dioxide, CO2, and a white powder, CaO.
a. By referring to the given, write down the chemical equation.
b. Compare the properties of the products and reactants in this reaction.
c. Use numbers of atoms to describe the amounts of calcium, carbon, and oxygen before and after the reaction.
d. Explain where the equation shows the conservation of matter.
e. Research another specific chemical reaction and create a poster to explain what happens during the reaction.
f. Design an experiment to test the reaction of two substances and record observations. Be specific about the substances and the observations.
Answers
Step-by-step Explanation:
a. \(CaCO_{3}\) → \(CaO\) + \(CO_{2}\)
This is the balanced chemical equation for the combustion of Calcium carbonate.
b. Properties of \(CaCO_{3}\) :
Odorless, white powderWater-insolubleCommon name - limestoneProperties of \(CaO\) :
Amorphous white solidSoluble in water and glycerolCommon name - quicklimeIonic bond between Calcium and OxygenProperties of \(CO_{2}\) :
One of the most popular greenhouse gasColorless, odorless, non-flammable gasWater-soluble - dissolves in water to form carbonic acidIt is 1.5 times heavier than airCooling of carbon dioxide liquid forms dry iceIt reacts with alkalis like NaOH to form carbonates which on further hydration gives bicarbonates.When passed through lime water, carbon dioxide turns limewater milky.c. According the above equation, number of atoms on both sides are equal.
Before reaction: Ca = 1, C = 1, O = 3
After reaction: Ca = 1, C = 1, O = 1 + 2 = 3
d. The law of conservation of matter states that the total mass and kind of elements in the reactants is equal to the total mass and kind of elements in the products. Thus matter cannot be created not destroyed, it can only be conserved. From the chemical equation, we get to know that the mass and number of atoms of all the elements is equal in the reactants and products. Thus we can prove the conservation of matter.
e. Combustion of Sodium Carbonate:
\(Na_{2} CO_{3}\) → \(Na_{2}O + CO_{2}\)
In this reaction, sodium carbonate undergoes combustion at temperature > 500°C and forms sodium oxide and carbon dioxide.
f. The following results are achieved when a chemical reaction occurs:
Change in stateChange in colorEvolution of a gasChange in temperatureWater and calcium oxide react quickly to form calcium hydroxide. A significant amount of heat is also released during this process, raising the system's temperature. This demonstrates that there was a chemical reaction.
Calcium carbonate breaks down into calcium oxide and carbon dioxide when heated. Here, the evolution of gas (carbon dioxide) proves that a chemical reaction has occurred.
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!
Answers
Answer:
\(MgO _{(s)} + 2H {}^{ + } _{(aq)} = Mg {}^{2 + } _{(aq)}+ H _{2} O _{(l)} \\ \)
A student increases the temperature of a 456 cm3 balloon from 255 k to 400 k.
assuming constant pressure, what should the new volume of the balloon be?
please hurry
Answers
The new volume of the balloon should be approximately 716.92 cm^3.
To find the new volume of the balloon, we can use the ideal gas law formula:
PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since the pressure is constant,
we can rewrite the formula as V1/T1 = V2/T2, where V1 is the initial volume, T1 is the initial temperature, V2 is the new volume we want to find, and T2 is the new temperature.
Given:
V1 = 456 cm^3
T1 = 255 K
T2 = 400 K
We can rearrange the formula to solve for V2:
V2 = V1 * (T2 / T1)
Substituting the given values, we have:
V2 = 456 cm^3 * (400 K / 255 K)
Calculating this expression:
V2 ≈ 456 cm^3 * 1.57
Therefore, the new volume of the balloon should be approximately 716.92 cm^3.
Learn more about volume in the link:
https://brainly.com/question/14197390
#SPJ11
which term describes an acid according to one acid base theory
Answers
A substance that contains a hydrogen atom and can be given as a hydrogen ion in an aqueous solution is considered an acid, according to the Arrhenius hypothesis. Arrhenius acids are the name given to such compounds.
According to Arrhenius hypothesis, what exactly is an acid?According to Arrhenius: Acid: Acids are those substances that, when dissolved in water, produce H+ ions. Base: Bases are compounds that, when dissolved in water, give off OH ions.
Which element falls under the definition of an H+ ion donor?According to the Bronsted-Lowry definition, an acid is a substance that contributes protons to the formation of a bond or takes a pair of valence electrons. (in the Lewis definition).
To know more about aqueous solution visit:-
https://brainly.com/question/26856926
#SPJ1
Question:
Which term describes an acid according to the Arrhenius acid-base theory?
Which substance has a melting point greater than room temperature
O A. argon
O B. oxygen
O
C. mercury
O D. aluminum

Answers
Aluminum is solid at room temperature and will, therefore, have a melting point that is above room temperature.
Melting pointThe room temperature is about 25 degrees Celsius.
Argon and oxygen are gases at room temperature.
Mercury is a liquid at room temperature.
Aluminum, on the other hand, is solid at room temperature. Hence, its melting point is definitely somewhere above room temperature.
More on melting points can be found here: https://brainly.com/question/25777663
How much heat is roguired to raise the temperature of 8.75 g of water from its melting point to its boiling pointsExpress your answer numerically in kilojoulos,
Answers
The heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
What exactly is specific heat?The amount of heat required to increase the temperature of one gram of a material by one degree Celsius (°C) is defined as specific heat.
What is the name of the specific heat formula?The equation q = mcΔt can be used to compute the amount of heat acquired or lost by a specific heat (q), where m is the mass of the sample, c is the specific heat, and Δt is the temperature change.
Given:
m = 8.75
c = 4.186 J/g°C
The melting point and boiling point of water is 0° and 100° respectively.
Δt = 100° - 0° = 100°
We know that,
q = mcΔt
= 8.75(4.186)100
= 3.662 kJ
Thus, the heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
Learn more about specific heat here:
https://brainly.com/question/21406849
#SPJ9
What is the oxidation number of s in na2s4o6?
Answers
Answer:
+2.5
Explanation:
So the oxidation number of S in Na2S4O6is +2.5.
Answer:
Explanation:
Oxidation number of Na = 1+ There are two of them, so it is 2*1 = 2
Oxidation number of O = 2- There are 6 of them so 6 * 2- = -12
There are 4 S's so the oxidation number is 4*S
Now set up a small equation
2 + 4s - 12 = 0
And solve.
2 - 12 + 4s =0
-10 + 4s = 0
4s = 10
4s/4 =10/4
s = 2.5
what molarity HCL solution forms from 0.915g HCL in 250mL of solution

Answers
Answer
0.1
Explanation
Given:
mass of HCl = 0.915g
volume of HCl = 250 mL
We know the molar mass of HCl = 36,458 g/mol
Solution
Step 1: covert the volume from mL to L
1 mL = 0.001 L
therefore 250 mL = 0.25 L
Step 2: Calculate the number of moles of HCl
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 0.915 g/36,458 g/mol
n = 0.025 mol
Step 3: Calculate the molarity
C = n/V where C is the molarity, n is the moles and V is the volume
C = 0.025 mol/0.25 L
C = 0.1
Select the correct answer. What is the hydronium (H3O+) concentration of a solution with a pH of 3.60?
Answers
Answer: The hydronium \((H_{3}O^{+})\) concentration of a solution with a pH of 3.60 is 0.56 M.
Explanation:
pH of a substance is the negative logarithm of concentration of hydrogen ions present in it.
It's formula is; pH = - log \([H^{+}]\)
When pH of a solution is 3.60 then its hydronium or hydrogen ion concentration is calculated as follows.
\(pH = - log [H^{+}]\\3.6 = - log [H^{+}]\\concentration of H^{+} = antilog (-3.6)\\= 0.56\)
Thus, we can conclude that the hydronium \((H_{3}O^{+})\) concentration of a solution with a pH of 3.60 is 0.56 M.
Which term describes a change that occurs with no new substances being formed?
A. Energy change
B. Bond change
C. Physical change
D. Chemical change
Answers
C. physical change
Explanation:
That's the answer
Scientists used to believe that chemical reactions fueled the sun. Why did this lead them to believe that the sun was very young? What kind of reactions do we now think are responsible for the energy released by the sun? In one to two sentences , explain your reasoning 2 points )
Answers
The energy produced by the sun is as a result of nuclear reactions which produces large amounts of energy.
What are chemical reactions?Chemical reactions are reactions which occur as a result of a rearrangement of atoms of elements to form new combinations of substances known as compounds.
Since chemical reactions which have relatively short lifespans, scientist would have assumed that the sun is very young.
However, nuclear reactions have been proven to be the means by which the sun produces its energy.
Nuclear reactions release large amounts of energy and have relatively long lifespans.
Therefore, the energy produced by the sun is as a result of nuclear reactions.
Learn more about nuclear reactions at: https://brainly.com/question/1272441
How many zeros are in the measurement 0.000040200 m are significant?
Answers
Answer:
It’s 3
Explanation:
low ph alkaline waves have a ph of