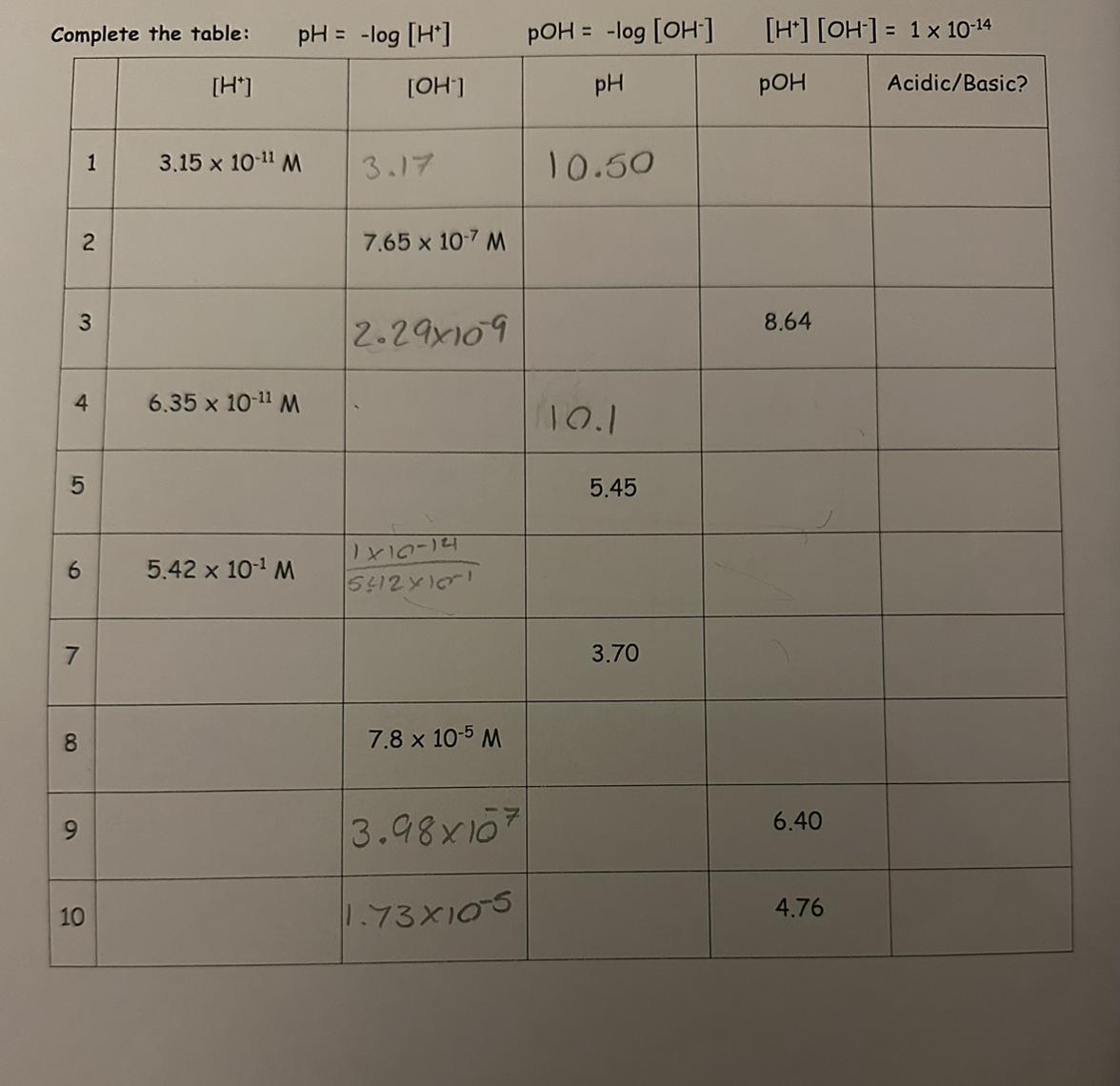
Answers
pH and pOH are two measures used to describe the acidity or alkalinity of a solution. pH represents the concentration of hydrogen ions (H+) in a solution, while pOH represents the concentration of hydroxide ions (OH-) in a solution. The relationship between pH and pOH can be described by the equation:
pH + pOH = 14
I will briefly explain pH and its relationship to acidity and alkalinity. pH is a logarithmic scale that ranges from 0 to 14, with values below 7 indicating acidity, values above 7 indicating alkalinity, and a pH of 7 representing neutrality. As the pH decreases, the acidity of the solution increases, meaning that the concentration of hydrogen ions (H+) increases. On the other hand, as the pH increases, the alkalinity of the solution increases, meaning that the concentration of hydroxide ions (OH-) increases. The pH scale is important in various scientific fields, including chemistry, biology, and environmental science, as it helps to determine the nature of solutions and their potential effects.
I will explain how to complete the table by calculating pH and pOH values for various solutions. To complete the table, you would need to determine the pH and pOH values based on the given information. For example, if the concentration of hydrogen ions (H+) is given, you can calculate the pH using the equation:
pH = -log[H+]
Similarly, if the concentration of hydroxide ions (OH-) is given, you can calculate the pOH using the equation:
pOH = -log[OH-]
Once you have calculated the pH and pOH values, you can complete the table accordingly. Remember to ensure that the sum of pH and pOH for each solution is equal to 14, as per the relationship mentioned earlier.
for more such questions on concentration
https://brainly.com/question/28564792
#SPJ8
Related Questions
Three separate companies produce lye, a strongly basic compound used mainly to produce soap and detergents. Each company uses a slightly
different method but charges the same amount per gram of theoretical yield. The company with the highest efficiency will be the cheapest to
purchase from. You work for Dirt-Be-Gone, a detergent company and must choose which lye company to give a contract to. You purchase one
unit of lye from each company to compare their efficiency.
Company 1: Theoretical yield of 12,000 grams, actually produces 8,476 grams per reaction.
Company 2: Theoretical yield of 9,000 grams, actualy produces 7892 grams per reaction.
Company 3: Theoretical yield of 7,000 grams, actually produces 4885 grams per reaction.
Who will you choose to provide your company with lye?
A. Company 3
B. Company 2
C. All are equal
D. Company 1
Answers
I'll choose Company 2 to provide my company with lye
Further explanationGiven
Company 1 : Theoretical yield :12,000 grams, actually 8,476 grams
Company 2 : Theoretical yield :9,000 grams, actually 7892 grams
Company 3 : Theoretical yield :7,000 grams, actually 4885 grams
Required
compare their efficiency and choose the company
Solution
To determine which company to choose, we must calculate how much percent yield each company produces. The greater the yield, the greater the efficiency. The company that has the most efficiency will be selected
% yield can be formulated :
\(\tt \%yield=\dfrac{actual}{theoretical}\times 100\%\)
Company 1\(\tt \%yield=\dfrac{8476}{12000}\times 100\%=70.63\%\)
Company 2\(\tt \%yield=\dfrac{7892}{9000}\times 100\%=87.69\%\)
Company 3\(\tt \%yield=\dfrac{4885}{7000}\times 100\%=69.79\%\)
Company 2 has the most efficiency
What are pr and vr called? is their use limited to isentropic processes?
Answers
Pr and Vr are dimensionless reduced pressure and reduced volume, respectively, and they are commonly used in thermodynamics to simplify the equations that describe the behavior of fluids. The use of Pr and Vr is not limited to isentropic processes.
Reduced pressure (Pr) is defined as the ratio of the actual pressure of a gas to its critical pressure, while reduced volume (Vr) is defined as the ratio of the volume of a gas to its critical volume. The critical point is the state at which a gas can no longer be liquefied by increasing its pressure at constant temperature.
These parameters are used in various thermodynamic relations, such as the compressibility factor, which describes the behavior of real gases. They are particularly useful when studying the behavior of fluids at different temperatures and pressures, as they allow for easy comparison of data for different substances.
Here you can learn more about thermodynamics
https://brainly.com/question/17074575#
#SPJ11
17. in which direction will the following equilibrium shift if a solution of ch3co2na is added?
Answers
The direction in which the equilibrium shifts when a solution of \(CH_3CO_2Na\) is added depends on the specific reaction and the role of acetate and sodium ions in that reaction.
When a solution of \(CH_3CO_2Na\) (sodium acetate) is added to a system in equilibrium, the equilibrium will shift in a specific direction depending on the reaction involved. However, without knowing the specific reaction or the initial conditions of the equilibrium, it is challenging to provide a definitive answer. In general, the addition of a sodium acetate solution can affect the equilibrium in multiple ways. Sodium acetate dissociates in water to produce acetate ions \((CH_3CO_2^-)\) and sodium ions (\(Na^{+}\)). These ions can interact with other species involved in the equilibrium, leading to various effects. If the equilibrium involves a reaction where acetate ions act as reactants, the addition of sodium acetate would increase the concentration of acetate ions in the solution. According to Le Chatelier's principle, this increase in concentration would shift the equilibrium towards the opposing direction to consume the excess acetate ions. Conversely, if the equilibrium involves acetate ions as products, the addition of sodium acetate would favor the forward reaction to maintain the equilibrium. Similarly, the addition of sodium ions can also affect the equilibrium. If the equilibrium involves a reaction where sodium ions act as reactants, the increase in their concentration would shift the equilibrium towards the products. On the other hand, if sodium ions are products, the addition of sodium acetate would not have a significant effect on the equilibrium position.
Learn more about equilibrium shifts here:
https://brainly.com/question/31387640
#SPJ11
Below is a chemical equation. Which of the following are identified by the blue arrows?
Captionless Image
atomic numbers
coefficients
products
subscripts

Answers
A spectrophotometer measures the transmittance or the absorbance, or both, of a particular wavelength of light after it has passed through a liquid sample. The liquid sample holder is commonly known as a cuvette. Before the transmittance or absorbance of the sample is measured, a cuvette filled only with solvent, called the blank, is placed in the spectrophotometer.
Answers
Option A is correct. Because a blank is measured to exclude the signal brought on by the cuvette walls and solvent, the blank was measured prior to the sample.
In order to identify a sample's fingerprint and calculate the amount of light reflected or transmitted from the various visible spectrum regions, spectrophotometers detect a sample's reflected or transmitted light. A spectrophotometer measures the amount of chemicals present in a solution or the amount of light absorbed. It determines how much of each color is present in a ray of light using the qualities of light and energy. A light source, optics to transmit the light, a detector, and a way to collect the light make up a spectrophotometer.
Therefore, option A is the best one.
The complete question is- Spectrophotometer measures the transmittance or the absorbance, or both, of a particular wavelength or light after it has passed through a liquid sample. The liquid sample holder is commonly known as a cuvette. Before the transmittance or absorbance of the sample is measured, a cuvette filled only with solvent, called the blank, is placed in the spectrophotometer. Select the statement from the following that is true for why is a blank measured before the sample.
A. a blank is measured to remove the signal caused by the cuvette walls and solvent.
B. a blank is measured to calibrate the spectrophotometer's light source.
C. there is no reason to measure a blank before the sample.
D. a blank is measured to clean the spectrophotometer.
Learn more about Spectrophotometer here-
https://brainly.com/question/24195565
#SPJ4
Which of the following reactions would have a decrease in entropy?
A. 2H20(g) → 2H2(g) + O2(9)
B. 2503(9) ► 2502(g) + O2(g)
C. 2NH3(9) ► N2(g) + 3H2(9)
D. 2NO2(g) → N204(9)
Answers
Answer:
D. 2NO2(g) → N204(9)
Explanation:
A p e x
2NO\(_2\)(g) → N\(_2\)O\(_4\)(g) reactions would have a decrease in entropy. Therefore, the correct option is option D.
What is entropy?Entropy is a measureable physical characteristic and a scientific notion that is frequently connected to a condition of disorder, unpredictability, or uncertainty. From classical thermodynamic, where it was originally recognized, towards the microscopic explanation of nature within statistical physics, the name and concept are employed in a variety of disciplines.
It has numerous applications in physics and chemistry, biological systems and how they relate to life, cosmology, economics, society, weather science, and information systems, especially the exchange of information. 2NO\(_2\)(g) → N\(_2\)O\(_4\)(g) reactions would have a decrease in entropy.
Therefore, the correct option is option D.
To know more about entropy, here:
https://brainly.com/question/14131507
#SPJ7
ANSWER QUICKLY PLEASE I GIVE BRAINLIEST


Answers
Answer:
characteristic of a good claim:
1. written in complete sentences
2. one sentence
3. answers one question
4. is stated as a fact
5. doesn't attempt to explain the claim
not a characteristic of a good claim:
1. multiple sentences
2. does not contain punctuation
3. answers multiple questions
4. starts with "i think"
5. attempts to explain the claim
6. uses weak words like "probably"
Explanation:
it is what it is
answer as much as you can please! need help :(

Answers
1. The number of moles of NaOH is 0.00162 moles
2. There are 0.00486 moles of citric acid
3. It is equivalent to 192 g of citric acid.
4. The mass of the citric acid is 12.95 g
What is neutralization?
1) The number of moles of the NaOH
Concentration * volume
= 0.1 M * 16.2/1000 L
= 0.00162 moles
1 mole of NaOH reacts with 3 moles of citric acid
0.00162 moles of NaOH reacts with 0.00162 * 3/1
= 0.00486 moles
Concentration of the citric acid = 0.00486 moles * 1000/25
= 0.19 M
Then;
m/M = CV
m = 0.19 * 355/1000 * 192
= 12.95 g
Learn more about neutralization:https://brainly.com/question/14156911
#SPJ1
draw the structure of a graphite
Answers

How do you solve this?? I will give brainliest for the right answer.

Answers
Wavelength of (nm) of photon emitted is 438nm
Wavelength is the distance between identical points means adjacent crest in the adjacent cycles of a waveform signal propagated in space or along a wire
Here in the given data is photon emitted when electron drop at 7th orbital means n = 7 and n = 1 for hydrogen atom and redberg constant is also given = 1.097×10⁷m⁻¹
we have to find wavelength = ?
Expression of wavelength of radiation is
1/λ = R (1/n₁² - 1/n₂²)
1/λ = 109677×(1/7²- 1/1²)
1/λ = 109677×48/49
λ = 438.69cm = 43869m
λ = 438nm
Know more about wavelength
https://brainly.com/question/14126236
#SPJ1
A gas expands from a volume of 4. 65 L to 6. 21 L under a constant external pressure of 2. 33 atm. How much work is done on the gas in joules?
Answers
To calculate the work done on the gas, we can use the formula:
Work (W) = -PΔV
Where:
W is the work done on the gas,
P is the constant external pressure, and
ΔV is the change in volume of the gas.
Given:
Initial volume (V1) = 4.65 L
Final volume (V2) = 6.21 L
External pressure (P) = 2.33 atm
First, we need to calculate the change in volume (ΔV):
ΔV = V2 - V1
ΔV = 6.21 L - 4.65 L
ΔV = 1.56 L
Now, we can substitute the values into the formula to calculate the work done on the gas:
W = -PΔV
W = -(2.33 atm) * (1.56 L)
Since we need the answer in joules, we need to convert atm·L to joules. The conversion factor is 101.325 J = 1 L·atm.
W = - (2.33 atm) * (1.56 L) * (101.325 J / 1 L·atm)
W = - 375.75 J
Therefore, the work done on the gas is approximately -375.75 joules. The negative sign indicates that work is done on the gas, as the volume increases under constant external pressure.
Learn more about gas here:
https://brainly.com/question/1312372
#SPJ11
Which statement provides the best explanation for the difference in heat energy required to melt and to boil water? Osheat is added, the molecules start to move faster and eventually break apart into the elements hydrogen and oxygen. The process begins in melting but is completed during boiling; therefore, boiling requires more energy than melting. O Molecules in liquid water are less tightly held than in the solid phase, while in the gas phase, no attractions exist between molecules. When changing from solid to liquid, the chemical bonds must weaken, but when changing from liquid to gas, these chemical bonds must be completely broken. Therefore, more energy is required to break the bonds completely and change i g of liquid water to 1 g of gaseous water Melting occurs at a lower temperature than boiling because in melting, solid water molecules become liquid water molecules, requiring less energy. However, in boiling, liquid water molecules break apart into hydrogen and coxygen gases, which requires significantly more energy O Molecules in liquid water are less tightly held than in the solid phase, while in the gas phase, no attractions exist between molecules. When changing from solid to liquid, the intermolecular forces must weaken, but when changing from liquid to gas, these intermolecular forces must be completely broken. Therefore, more energy is required to break the intermolecular forces completely and change 18 of liquid water to 1 g of gaseous water.
Answers
Heat energy, also known as thermal energy, is a form of energy that is transferred between objects or systems as a result of temperature differences. When water changes from a solid to a liquid (melting), the intermolecular forces weaken, but they are not completely broken. On the other hand, when water changes from a liquid to a gas (boiling), the intermolecular forces must be completely broken.
The statement that provides the best explanation for the difference in heat energy required to melt and to boil water is: "Molecules in liquid water are less tightly held than in the solid phase, while in the gas phase, no attractions exist between molecules. When changing from solid to liquid, the intermolecular forces must weaken, but when changing from liquid to gas, these intermolecular forces must be completely broken. Therefore, more energy is required to break the intermolecular forces completely and change 18 of liquid water to 1 g of gaseous water." This means that the process of boiling requires more energy than melting because in boiling, the intermolecular forces between liquid water molecules must be completely broken to transform into gaseous water, which requires more energy than weakening the intermolecular forces in melting solid water into liquid water.
Learn more about intermolecular forces here ;
https://brainly.com/question/32203220
#SPJ11
Why do ionic compounds need to reach stability in their charges?
Answers
Answer:
Ionic compounds need to reach stability in their charges because they are composed of ions, which are atoms or molecules that have gained or lost electrons. The positive and negative charges within an ionic compound must balance out in order to achieve stability. If the charges are not balanced, the compound will be unstable and will tend to react with other ions or molecules in an attempt to reach a state of stability. This is why ionic compounds form crystal lattices, with each ion arranged in a specific position in the lattice so that the positive and negative charges are evenly balanced.
Explanation:
Tell me if you still confuse
ALLEN
how long ago did two organisms that show 20 differences in their genetic sequences diverge?
Answers
Answer:
Comparison of whole genome sequences provides a highly detailed view of how organisms are related to each other at the genetic level.
Explanation:
A 50.0 mL quantity of a 0.20 M solution of one of the following weak bases is titrated with 0.050 M HCl. At the equivalence point, the pH is 2.99. Identify the weak base. a. ethylamine (k_b = 5.6 Times 10^-4) b. methylamine (k_b = 4.4 Times 10^-4) c. ammonia (k_b = 1.8 Times 10^-5) d. pyridine (k_b = 1.7 Times 10^-9) e. aniline (k_b = 3.8 Times 10^-10) f. urea (k_b = 1.5 Times 10^-14)
Answers
The value of Kb for aniline is 3.98 x 10⁻¹⁰. So the correct option is E.
At the equivalence point :
No. of moles of acid = No. of moles of base
Molarity (M1) x Volume (V1) for acid = Molarity (M2) x Volume (V2) for base.
Given, V2 = 50.0 mL,
M2= 0.20 M,
M1 = 0.05 M
V1 = M2xV2/M1
V1 = 50.0 mL x 0.20 M / 0.05 M
V1 = 200 mL
Now,
pOH = 1/2 [ pKw + pKb + log C]
14 - pH = 1/2 [ pKw + pKb + log C]
11.01 = 1/2 [ 14 + pKb + log 0.010 mol / 0.250 L ]
As, no. of moles = 0.050 L x 0.2 M = 0.010 mol and Total volume = 250 mL = 0.250 L
22.02 = 14 + pKb + log 0.04
8.01 = pKb - 1.398
pKb = 8.01 + 1.398
pKb = 9.4
Kb = 10-pKb
Kb = 10-9.4
Kb = 3.98 x 10⁻¹⁰ (for aniline).
To learn more about aniline, refer to the link:
https://brainly.com/question/32043569
#SPJ12
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
What is a main-sequence star's estimated luminosity that has strong ionized calcium absorption lines and weak hydrogen absorption lines?
Answers
A main-sequence star's estimated luminosity with strong ionized calcium absorption lines and weak hydrogen absorption lines can be determined by comparing its position on the HR diagram to known stars.
The estimated luminosity of a main-sequence star with strong ionized calcium absorption lines and weak hydrogen absorption lines can vary depending on the specific characteristics of the star. These spectral features provide clues about the star's temperature, composition, and evolutionary stage.
One possible explanation for a star with strong ionized calcium absorption lines and weak hydrogen absorption lines is that it is an early-type star, such as a B or A type star. These stars are hotter and more massive than the Sun. They have strong ionized calcium lines because they have higher temperatures, which cause more calcium atoms to become ionized. On the other hand, their weak hydrogen absorption lines suggest that their outer layers contain relatively less hydrogen compared to other main-sequence stars.
To estimate the luminosity of such a star, we can use the Hertzsprung-Russell (HR) diagram. The HR diagram plots a star's luminosity against its temperature. By comparing the star's position on the HR diagram to the positions of known stars, we can estimate its luminosity.
For example, if we find a star with strong ionized calcium absorption lines and weak hydrogen absorption lines located in the upper-left portion of the HR diagram, it would indicate that the star is more luminous than the Sun. Conversely, if the star is located in the lower-right portion of the HR diagram, it would suggest a lower luminosity.
Keep in mind that estimating a star's luminosity based solely on its spectral features is not always precise. Other factors, such as distance and interstellar extinction, can affect the observed luminosity. Additionally, it's important to consider that multiple scenarios and classifications are possible based on the information provided in the question.
However, it's important to consider other factors that may affect the observed luminosity.
Learn more about absorption lines here:-
https://brainly.com/question/31230822
#SPJ11
Someone plz help me ;-;

Answers
Answer:
the events of the story
Explanation:
hope this helps
How many moles of sulfur
dioxide are in 2.26 x 10^33 sulfur dioxide molecules?
Answers
Answer:
moles = no. of molecules / Avogadro's number
= 2.26 x 10^33 / 6.022 x 10^23
= 3752906011
Round to significant figures which is 3 = 3.75 x 10^9 mol
Explanation: The formula for finding how many moles of a substance when given the amount of molecules is: moles = number of molecules / Avogadro's number
Which of the following is an element? aluminum bronze table salt brass
Answers
Answer:
Aluminum is an element
Explanation:
Aluminum is the most abundant metal in the earths crust with an atomic number of 13.
Can someone please help me with science.

Answers
Answer:
The tendency of an object to resist changes in its state of motion varies with mass. Mass is that quantity that is solely dependent upon the inertia of an object. The more inertia that an object has, the more mass that it has. A more massive object has a greater tendency to resist changes in its state of motion.
which of the following best describes a single replacement reaction?
two elements combine to form a compound
one element takes the place of another in a compound
two elements switch places in a compound
a compound breaks into separate elements
Answers
Answer:
One element takes the place of another in a compound
Explanation:
I just took a test for it and got it right. :)
Hope This Helps :)
you are asked to prepare 100ml of a 1.5m kbr solution. what mass of kbr do you need? show your calculation in the space provided.
Answers
To prepare 100ml of a 1.5m kbr solution. To find mass of kbr we need
we can use the formula:
mass = molarity x volume x molar mass
where molar mass of KBr is 119.00 g/mol.
So,
mass = 1.5 M x 100 ml x (119.00 g/mol) / 1000 ml/L
mass = 1.785 g
Therefore, 1.785 g of KBr is required to prepare 100 ml of a 1.5 M solution.
Potassium Bromide, sometimes known as KBr, is a salt that is commonly used as an anticonvulsant and sedative.
Other names for potassium bromide include Kalii bromidum, tripotassium tribromide, and bromide salt of potassium.
The odourless potassium bromide salt has a sharp, bitter salty flavour and is available as white crystals, colourless crystals, or white granular solids. Aqueous KBr solutions have a pH of 7.
Learn more about Potassium Bromide here :
brainly.com/question/17154705
#SPJ4
a physiciist is studying the nature of static discharge by appying a volatge across a microscopic tube filled with nitrogen
Answers
The process that correctly describes the growth in the number of ionized nitrogen molecules over time is D. The number of ionized molecules increases exponentially with each ionized molecule ionizing 3 other molecules every nanosecond.
Let's analyze the equation tN2 = 3⋅N, where N is the number of ionized molecules and t is the time in nanoseconds.
The equation states that the number of ionized molecules at time t is equal to 3 times the number of ionized molecules at the previous time step.
This implies that each ionized molecule ionizes 3 other molecules every nanosecond.
A physicist is studying the nature of static discharge by applying a voltage across a microscopic tube filled with nitrogen.
Static discharge is an event that takes place when there is a sudden flow of electric charge between two objects with different electrical potentials. This electric charge transfer results in a brief burst of electromagnetic energy (EMI or EMF), which can sometimes be observed as a visible spark in low-light conditions or heard as a crackling sound in high-quality audio systems.
The nature of static discharge is dependent on a variety of factors, including the composition of the material, the temperature and humidity, the electrical potential between the two objects, and the distance between the objects. Typically, static discharge occurs more frequently in environments with low humidity, such as during the winter months or in arid regions, as moisture acts as an insulator and prevents the buildup of electrical charge. In addition, materials that are good electrical conductors, such as metals, are more likely to experience static discharge than materials that are poor conductors, such as plastics or rubber.
Therefore, the correct description of the growth in the number of ionized nitrogen molecules over time is:
D. The number of ionized molecules increases exponentially with each ionized molecule ionizing 3 other molecules every nanosecond.
Learn more about static discharge here: https://brainly.com/question/30326702
#SPJ11
Complete question is:
" physicist is studying the nature of static discharge by applying a voltage across amicroscopic tube filled with nitrogen molecules.Every nanosecond from then on, any ionized molecules willionize an additional number of molecules not already ionized, and ionization does not get lost.The equationtN2 3shows N, the number of ionized molecules, t nanoseconds after initiating a voltage.Which of thefollowing correctly describes the growth in the number of ionized nitrogen molecules over time?A.The number of ionized molecules increases linearly with each ionized molecule ionizing 2 other moleculesevery nanosecond.B.The number of ionized molecules increases linearly with each ionized molecule ionizing 3 other moleculesevery nanosecond.C.The number of ionized molecules increases exponentially with each ionized molecule ionizing 2 othermolecules every nanosecond.D.The number of ionized molecules increases exponentially with each ionized molecule ionizing 3 othermolecules every nanosecond."
What is the relationship between osmolarity and water activity?
(A) There is a negative correlation; as osmolarity increases water activity also increases.
(B) There is a positive correlation; as osmolarity increases water activity decreases.
(C) There is no correlation between osmolarity and water activity.
(D) There is a negative correlation; as osmolarity increases water activity decreases.
(E) There is a positive correlation; as osmolarity increases water activity also increases.
Answers
Osmolarity refers to the concentration of solutes in a solution, while water activity represents the availability of water molecules for biological reactions. The correct answer is (D) There is a negative correlation; as osmolarity increases, water activity decreases.
As the osmolarity of a solution increases, it means there are more solutes present, resulting in a lower water activity.
Higher solute concentration reduces the amount of free water molecules, making water less available for biological processes.
Therefore, there is a negative correlation between osmolarity and water activity. The correct option is D.
To know more about Osmolarity, refer here:
https://brainly.com/question/32470302#
#SPJ11
Choose the correct products for the double replacement reaction below. Click here to access the solubility rules to determine which product, if any, forms a solid precipitate in the reaction. AgNO3 KCl Upper K l Upper Pb (Upper N Upper O Subscript 3) Subscript 2 Baseline right arrow. ? K O2 NCl KNO3 AgCl AgK ClNO3 K2NO3 AgCl2.
Answers
In both cases, the precipitates were AgCl and PbI2 respectively.
What is a precipitate?A precipitate is a solid product obtained from the reaction of two aqueous phase species. Let us now consider the two reactions listed in the question;
AgNO3(aq) + KCl(aq) ----> AgCl(s) + KNO3(aq)2KI(aq) + Pb(NO3)2 (aq) ----->PbI2(s) + 2KNO3(aq)We can see that in both cases, the precipitates were AgCl and PbI2 respectively.
Learn more about precipitates: https://brainly.com/question/24846690
Answer:
B and B
Explanation:
edge 2022
for each of the reactions at constant pressure, determine whether the system does work on the surroundings, the surroundings does work on the system, or essentially no work is performed.
Answers
Answer:
It is not possible to accurately determine whether the system or the surroundings performs work in a chemical reaction without more information about the specific reaction and the conditions under which it occurs.
In general, the work done in a chemical reaction can be affected by several factors, including the pressure, volume, and temperature of the system, as well as the nature of the reactants and products. Some reactions may result in the system doing work on the surroundings, such as when gases expand and do work on their surroundings by pushing against a piston or other external object. Other reactions may result in the surroundings doing work on the system, such as when gases are compressed and their surroundings do work on them. Still other reactions may result in essentially no work being performed, such as when the reactants and products are in equilibrium or when the volume of the system remains constant.
Without more information about the specific reaction in question, it is not possible to accurately determine whether the system or the surroundings performs work.
Predict the products of the following single replacement reaction and predict whether it will be spontaneous. Cu(s) + ZnCl2(aq) + If the reaction is not spontaneous choose "No reaction occurs (not spontaneous)" A. No reaction (i.e., not spontaneous) B. CuCl2(aq) + Zn(s) C. Cu(s) + Zn(s) D. ZnCl2(aq) + CuCl2(aq)
Answers
△G= △H - T △S
Where △G is change in Gibbs free energy
△H is change in enthalpy
△S is entropy change .
Any reaction is spontaneous if △G is negative for that reaction.
T is 273K (standard conditions)
△G = -219000 - 273 ×(- 21)
△G = -213267 J
Since △G is negative hence reaction is spontaneous .
What is the change in enthalpy?
The enthalpy change is approximately equal to the difference between the energy used to break bonds in a chemical reaction and the energy gained by forming new chemical bonds in the reaction. It describes the change in energy of a system at constant pressure. The enthalpy change is denoted by ΔHTo know more about change in enthalpy, click the link given below:
https://brainly.com/question/29556033
#SPJ4
CuCl2(aq) + Zn(s) reaction and predict whether it will be spontaneous.
What is reaction?
Reaction is the process of responding to a stimulus. It could be a physical response, such as an increase in heart rate or a change in body temperature. It could also be a mental response, such as a change in thoughts or emotions. Reactions can be conscious or unconscious, and can vary from person to person. They can be short-term or long-term, depending on the situation. Reactions are important for survival as they help us respond quickly to changes in our environment. They can also be used to learn and adapt to new situations.
This single replacement reaction is spontaneous because copper is more reactive than zinc, meaning copper will replace zinc in the solution. The products of this reaction will be CuCl2(aq) and Zn(s).
To know more about reaction
https://brainly.com/question/25769000
#SPJ4
What is the value of R in the ideal gas law?
O A. -0.0821 L'atm/mol K
OB. 0.0821 L'atm/mol:K
O c. 273 L'atm/mol K
O D. -273 L'atm/mol K
Answers
does The Bohr model of the atom correctly predicts the energy levels of the hydrogen atom, which has a single electron.
Answers
Answer:False. The Bohr model correctly predicts energy levels of hydrogen and hydrogen-like atoms.
Explanation: