period 6, group 12 (2B)
Answers
Answer:
mercury or Hg
Explanation:
i looked at the periodic table haha
hope this helps and is right <3
Related Questions
Find the molar mass of AI(NO3)3?
Explain plz!
Answers
Answer:
212.996 or 213 approx.
hope it helps.
Molar mass of Al - 27
Molar mass of N - 14
Molar mass of O - 16
so, the molar mass of (No3)3 will be (14+16×3)3 which is equals to 186.
so, Molar mass of Al(No3)3 will be 186+27 = 213.
Answer:
\(\boxed {\boxed {\sf 212.99 \ g/mol}}\)
Explanation:
The molar mass is the mass of 1 mole of a substance. These values are found on the Periodic Table because they are equal to the atomic masses, but the units are grams per mole instead of atomic mass units.
Aluminum nitrate is made up of 3 elements: aluminum, nitrogen, and oxygen. Look up the molar masses of the individual elements.
Al: 26.981538 g/mol N: 14.007 g/mol O: 15.999 g/molLet's analyze the chemical formula: Al (NO₃)₃
NO₃ is in parentheses with a subscript of 3 after. This means there are 3 nitrate ions in 1 mole of aluminum nitrate. Find the molar mass of a nitrate ion, multiply by 3, then add aluminum's molar mass.
NO₃ = 14.007 + 3(15.999) = 14.007+47.997 =62.004 g/mol (NO₃)₃ = 3(62.004) =186.012 g/mol Al (NO₃)₃ = 26.981538 + 186.012 = 212.993538 g/molWe can round to the nearest hundredth. The 3 in the thousandth place tells us to leave the 9 in the hundredth place.
Al(NO₃)₃ ≈ 212.99 g/molThe molar mass of aluminum nitrate is approximately 212.99 grams per mole.
one ball of lead has a volume of 90 cm and a mass of 1,017g. a larger ball of lead has a volume of 250 cm and a mass of 2,825g. complete the calculations and state whether or not the density changes.
Answers
The density of the balls, given the masses and volume of each balls did not change
How do I know whether or not the density changes?To Know if there is a change or not, we shall determine the density of both lead balls. Details below:
For the small ball
Mass of small ball = 1017 gVolume of small ball = 90 cm³Density of small ball = ?Density = mass / volume
Density of small ball = 1017 / 90
Density of small ball = 11.3 g/cm³
For the larger ball
Mass of larger ball = 2825 gVolume of larger ball = 250 cm³Density of larger ball = ?Density = mass / volume
Density of larger ball = 2825 / 250
Density of larger ball = 11.3 g/cm³
From the above calculations, we obtained:
Density of small ball = 11.3 g/cm³Density of arger ball = 11.3 g/cm³Thus, we can conclude that the density of the lead balls did not change
Learn more about density:
https://brainly.com/question/952755
#SPJ1
What is the atomic mass of Carbon?
Answers
Answer:
Ar = 12
Explanation:
I think at GCSE level it's 12. It might be more specific in A-Level and further.
Answer:
atomic mass of carbon is
12.0107 u mark me as brainlist
nitrogen monoxide and oxygen react to form nitrogen dioxide. if 5.6 moles of no react with 3.1 moles of o 2, how many moles of the reactant in excess will remain after the reaction? (assume 100% yield.)
Answers
0.3 moles of Oxygen is excess reactant (reagent) when yield is 100% .
NO =5.6 moles
O2= 3.1 moles
2NO + O2 --> 2NO2 the chemical reaction.
the ratio of NO and O2 is 2:1
therefore for 5.6 moles of NO, 2.8 moles of Oxygen(O2) is required. So 3.1moles - 2.8 moles = 0.3 moles
The reactant in a chemical reaction that is present in a quantity that is larger than what is required to fully react with the limiting yield is known as the excess reactant. The substance(s) still present after a chemical reaction has reached equilibrium in yield are the reactant(s). Subtract the mass of excess reagent eaten from the total mass of excess reagent provided to determine the quantity of yield that is still present. Reactants in a chemical reaction are referred to as excess reagents if they are still present after the reaction is complete.
learn more about yield here:
https://brainly.com/question/25996347
#SPJ4
*
When Gallium becomes an ion it loses 3 electrons to become:
A: -3
B: +3
C: It loses 3 electrons to become a neutral atom
D: It is already neutral
Answers
Answer:
B: +3
Explanation:
If Gallium loses 3 electrons, it will become an ion.
The ion will be positively charged because in this new ion, the number of electrons is lesser than the number of protons. The charge difference will impart a positive net charge on the ion.
In a neutral atom, the number of electrons and protons are the same. For positively charged ions, the number of protons is greater than the electronsIf Gallium the loss of 3 electrons offsets the charge balance in the chemical specie. Thus, the ion will have a net +3 charge.
7. What's the mass of 60 mL of oil if the oil has a density of 0.90 g/mL?
A. 67 g/ml.
B. 54 g/mL
C..02 g/mL
D. 5.4 g/mL
Answers
The mass of 60 mL of oil is 54 g.The required answer is 54 g.
correct option is B
The given density of oil is 0.90 g/mL. Also, given that the volume of oil is 60 mL. We are required to find out the mass of 60 mL of oil, given that the density of oil is 0.90 g/mL.The density of an object is defined as the amount of mass present per unit volume of the object.
The formula to calculate the mass of an object, given its volume and density is as follows: Mass = Density × Volume Substituting the given values in the above formula, we get:Mass = 0.90 g/mL × 60 mL= 54 g.
correct option is B
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
How many protons, neutrons, and electrons does an atom of Carbon-13 contain? Carbon has an atomic number of 6.
A) 6 protons, 6 electrons, and 13 neutrons
B) 6 protons, 7 electrons, and 7 neutrons
C) 6 protons, 6 electrons, and 7 neutrons
D) 13 protons, 6 electrons, and 6 neutrons
Answers
Answer:
B I think
Explanation:
Answer:
C) 6 protons, 6 electrons, and 7 neutrons.
Explanation:
The number of protons in an atom is called the atomic number, and it is unique to each element. Since carbon has an atomic number of 6, every atom of carbon will have 6 protons.
Carbon-13 is a variant of carbon that has an additional 7 neutrons in its nucleus, but the number of protons and electrons remains the same as any other atom of carbon. Therefore, an atom of Carbon-13 will have 6 protons, 6 electrons, and 7 neutrons.
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Assuming the volume of a gas in a closed system is constant. If the pressure is increased, how can the system adjust to the change?
Answers
Answer:
Decrease the temperature in the system
Please help, this assignment is to hard for me. :(

Answers
Answer:
603000 J
Explanation:
The following data were obtained from the question:
Energy required (Q) =...?
Mass (M) = 10000 g
Specific heat capacity (C) = 2.01 J/g°C
Overheating temperature (T2) = 121°C
Working temperature (T1) = 91°C
Change in temperature (ΔT) =.?
Change in temperature (ΔT) =T2 – T1
Change in temperature (ΔT) = 121 – 91
Change in temperature (ΔT) = 30°C
Finally, we shall determine the energe required to overheat the car as follow:
Q = MCΔT
Q = 10000 × 2.01 × 30
Q = 603000 J
Therefore, 603000 J of energy is required to overheat the car.
Even before modern observations provided evidence supporting the theory of plate tectonics, people developed theories that the continents were once joined together. Using only maps, what did they observe?
Answers
Answer:
people or scientist tend to observe the nature of the continents that described by jigsaw fit,geological similarity among the continents
The OH-ion concentration of a blood sample is
2.5 x 10-7M. What is the pH of the blood?
a. 6.60
b.0.99
c.740
d.1
Answers
Answer:
She okeyy
Explanation:
Example 740 are the think free ofıce pH of the blood 740 blood sample iş takipte kal ❣️
What has more mass 100 apples or 100 grapes? Why?
Answers
Answer:
Apples weigh more due to the larger mass, yet same quantity. With a 1050% increase over an average grape. Multiply 150g x 7g = 15000, bow multiply 7g x 100g = 700. Both equal
Explanation:
Your average apple weighs about 150g and 250g. The average grape weighs about 7g being small, and a large 11g. That's a 1050% increase.. Disclaimer I haven't measured massxweightxheight in awhile so might be completely wrong Mass is a measure of how much force it will take to chanfe the path
Answer:
Your average apple weighs about 150g and 250g. The average grape weighs about 7g being small, and a large 11g. That's a 1050% increase.. Disclaimer I haven't measured massxweightxheight in awhile so might be completely wrong Mass is a measure of how much force it will take to chanfe the path
Explanation:
Name this compound. Please help
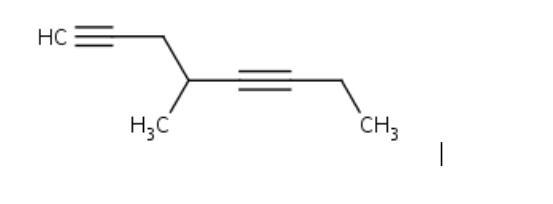
Answers
Answer:
mitosis
Explanation:
The shape of the methane molecule(ch4) is called
Answers
Answer:
Tetrahedral
Explanation:
calculate the ph of a buffer solution prepared by mixing 30 ml of 5% acetic acid with 10 ml of 0.5 m naoh.
Answers
5 g of acetic acid are present in 100 g of solution, or 5% acetic, implies.
5 g is the mass of acetic acid.
Acetic acid's molar mass is 60 g/mol.
Mass divided by molar mass yields acetic acid moles.
= 5 / 60
= 0.0833
Acetic acid's molarity is equal to moles per volume.
= 0.0833 / 30 x 10^-3
= 2.78 M
0.005 moles of NaOH are equal to 10 x 0.5 / 1000.
NaOH + CH3COOH —————————-> CH3COONa + Water
0.0833 0.005 0 - ———————-> initial
After reaction,
0.0783 0 0.005
pH is calculated as [CH3COONa/CH3COOH] log pKa.
pH = 4.74 + log (0.005 / 0.0783)
pH = 3.54
A buffer is a substance that can withstand a pH change when acidic or basic substances are added. Small additions of acid or base can be neutralized by it, keeping the pH of the solution largely constant. This is crucial for procedures and/or reactions that call for particular and stable pH ranges.
To know more about buffer solution, click on the link below:
https://brainly.com/question/22390063
#SPJ4
what is the density of a 416.2 g rock that has a volume of 32cm3
Answers
Answer:
13g/cm"^3
Explanation:
Density=mass/volume.
D=416.2/32
D=13.006
D= 13 g/cm^3
Which of these is true for a molecular model like the one shown ? A. We can always identify the type of each atom based on color. B. The model shows how the various atoms are connected in the molecule. C. The model shows the actual size of each atom correctly D. The model shows the relative sizes of the atoms to each other.
Answers
The true statement about the model is that the model shows how the various atoms are connected in the molecule.
What is a molecular model?A molecular model is a representation of a molecule. We know that the molecular model shows the extent to which the atoms are bonded in the compound. The model gives us an idea of the intricate atom to atom arrangement that exists in the molecule that is under study.
Now we know that the atoms that are in the models must be shown by the use of different colors. This enables us to be able to effectively and properly distinguish between the atoms of the compound as shown in the model.
The true statement about the model is that the model shows how the various atoms are connected in the molecule.
Learn more about molecular models:https://brainly.com/question/156574?
#SPJ1
the reaction of 1-bromobutane with sodium hydroxide affords the substitution product 1-butanol. what would happen to the rate of the reaction if the concentration of both 1-bromobutane and sodium hydroxide were doubled?
Answers
The rate increases by a factor of 4. IF the rate of the reaction concentration of both 1-bromo butane and sodium hydroxide were doubled
What is meant by rate of reaction?
The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction.The reaction rate or rate of reaction is that the speed at which a chemical reaction takes place, defined as proportional to the rise in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically
Why is rate of reaction important?
The rate of a reaction is a powerful diagnostic tool. By checking out how fast products are made and what causes reactions to slow down we can develop methods to improve production. This information is important for the large scale manufacture of many chemicals including fertilizers, drugs and household cleaning items.
Learn more about rate of reaction :
brainly.com/question/25724512
#SPJ4
Để trung hoà 50 ml hỗn hợp X gồm HCl và H2SO4 cần dùng 20 ml dung dịch NaOH 0,3M. Cô cạn dung dịch sau khi trung hoà thu được 0,381 gam muối khan. a) Xác định nồng độ mol của các axit trong X. b) Tính pH của dung dịch X.
Answers
Answer:
Để trung hoà 50 ml hỗn hợp X gồm HCl và H2SO4 cần dùng 20 ml dung dịch NaOH 0,3M. Cô cạn dung dịch sau khi trung hoà thu được 0,381 gam muối khan. a) Xác định nồng độ mol của các axit trong X. b) Tính pH của dung dịch X.
please help quick the 6th question is “What is a catalyst?” (include all three parts)

Answers
2. I know this because the delta H is a negative number. (-802.4 kJ)
3. This means that this reaction releases heat.
A sample of gas at a fixed pressure has a temperature of 300 K and a volume of 3 L. Calculate the volume if the gas is heated to 700 K.
Answers
Answer:300k=3L
700k=x
cross multiply
300x=2100; x=7L
Explanation:
Answer:
7L
Explanation:
a sample of unknown material weighs 500 n in air and 200 n when immesersed in alcholol with a specfic gravity of 0.7 what is the mass density
Answers
Answer: The mass density is 1166.36 \(kg/m^{3}\).
Explanation:
Given: Weight of sample in air \((F_{air})\) = 500 N
Weight of sample in alcohol \((F_{alc})\) = 200 N
Specific gravity = 0.7 = \(0.7 \times 1000 = 700 kg/m^{3}\)
Formula used to calculate Buoyant force is as follows.
\(F_{B} = F_{air} - F_{alc}\\= 500 - 200 \\= 300 N\)
Hence, volume of the material is calculated as follows.
\(V = \frac{F_{B}}{\rho \times g}\)
where,
\(F_{B}\) = Buoyant force
\(\rho\) = specific gravity
g = acceleration due to gravity = 9.81
Substitute the values into above formula.
\(V = \frac{F_{B}}{\rho \times g}\\= \frac{300}{700 \times 9.81}\\= \frac{300}{6867}\\= 0.0437 m^{3}\)
Now, mass of the material is calculated as follows.
\(mass = \frac{F_{air}}{g}\\= \frac{500 N}{9.81}\\= 50.97 kg\)
Therefore, density of the material or mass density is as follows.
\(Density = \frac{mass}{volume}\\= \frac{50.97 kg}{0.0437 m^{3}}\\= 1166.36 kg/m^{3}\)
Thus, we can conclude that the mass density is 1166.36 \(kg/m^{3}\).
a reaction has a δhrxn = 23.25 kj and δs was 161.26 j/mol∙k. this reaction is spontaneous
Answers
The spontaneity of a reaction is determined by the Gibbs free energy change (∆G), which is a measure of the system's ability to do work.
The equation that links ∆G, ∆H, and ∆S is: ∆G = ∆H - T∆S, where T is the temperature in Kelvin and ∆H and ∆S are the enthalpy and entropy changes, respectively. The signs of ∆H and ∆S determine whether the reaction is exothermic or endothermic and whether it is entropy-driven or enthalpy-driven, respectively.
If ∆G is negative, the reaction is spontaneous, meaning it occurs without the input of energy.The given reaction has a ∆H of 23.25 kJ and a ∆S of 161.26 J/mol∙K.
First, we need to convert the units of ∆S from J/mol∙K to kJ/mol∙K by dividing by 1000.∆S = 161.26 J/mol∙K ÷ 1000 = 0.16126 kJ/mol∙K Substitute the values into the equation to determine the spontaneity of the reaction:
∆G = ∆H - T∆S∆G = (23.25 kJ) - (298 K) x (0.16126 kJ/mol∙K)∆G = 23.25 kJ - 48.02 kJ∆G = -24.77 kJ Since ∆G is negative, the reaction is spontaneous.
To know more about Gibbs free energy refer here: https://brainly.com/question/13795204#
#SPJ11
What reaction is used to remove one phosphate group from ATP?A. hydrolysis reactionB. redox reactionC. combustion reactionD. neutralization reaction

Answers
Answer:
A. hydrolysis reaction.
Explanation:
Chemical Reactions.
First, let's review each concept of the group of answer choices:
- hydrolysis: is a reaction in which the net reaction is an organic compound reacting with water to give either two molar equivalents of a single product or more than one product.
- redox: is a type of chemical reaction that involves a transfer of electrons between two species.
- combustion: is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
- neutralization: is a reaction that occurs when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water.
The problem is asking for the reaction that removes a phosphate group from ATP, so let's see the structure of ATP with one phosphate group:
What is enclosed in the red box is the phosphate group.
The reaction that removes this phosphate group represents a rupture of the structure and based on the logic of the definitions of the given concepts, the answer would be that the reaction to remove one phosphate group from ATP is A. hydrolysis reaction. This reaction looks like this:
ATP + water (H2O) -> ADP + Pi,
where ADP is the same molecule of ATP but it has two phosphate groups and Pi is the phosphate group removed.

3KOH + FeCl3 → Fe(OH)3 + 3KCl
How many grams of KCl are produced when 12.5 moles of KOH completely react with excess of FeCl3?
Answers
Answer:
sry, dk
Explanation:
11.All of the following properties of a diamond are physical except...Select one:a. It does not conduct electricity.b. It produces carbon dioxide when burned in pure oxygen.c. It is transparent like glass.d. It is the hardest material.
Answers
Answer
b. It produces carbon dioxide when burned in pure oxygen.
Explanation
The reaction between diamond and oxygen, producing carbon dioxide is not a physical property of diamond, it is a chemical property because breaking and synthesis of chemical bonds occur.
PLEASE HELP! Please answer thanks

Answers
Answer:
4m
Explanation:
wavelength is peak to peak and 4m is the closest
What is the specific kind of product that is formed in the reduction of ketones?
Answers
The specific kind of product that is formed in the reduction of the ketone is the alcohol functional group.
What is the reduction of ketones?When ketone reacts with the silver oxide it forms the alcoholic group and a silver mirror white precipitate which indicated the reduction of ketones.
With other like lithium aluminum hydride or sodium Bromo hydride it gets also reduced to form the product alcohol.
Therefore, a specific kind of product that is formed in the reduction of the ketone is the alcohol functional group.
Learn more about the reduction of ketones, here:
https://brainly.com/question/24065591
#SPJ4
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy (ΔH°) for the given reaction is 235.8 kJ/mol.
The standard enthalpies of formation (H°f) of the associated reactants and products must be taken into account in order to get the standard reaction enthalpy (H°) for the given reaction.
The balanced equation is:
3Fe₂O₃(s) → 2Fe3O₄(s) + ½O₂(g)
The standard enthalpy of formation values for Fe2O3(s), Fe3O4(s), and O2(g) are required.
The values are:
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe3O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
Now, find the standard reaction enthalpy by using equation:
ΔH° = ΣnΔH°f(products) - ΣnΔH°f(reactants)
In which n is the stoichiometric coefficient of each substance.
Place the values, to get:
ΔH° = [2ΔH°f(Fe3O₄) + ½ΔH°f(O2)] - [3ΔH°f(Fe₂O₃)]
ΔH° = [2(-1118.4 kJ/mol) + ½(0 kJ/mol)] - [3(-824.2 kJ/mol)]
ΔH° = [-2236.8 kJ/mol] - [-2472.6 kJ/mol]
ΔH° = -2236.8 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Thus, the standard reaction enthalpy (ΔH°) for the given reaction is 235.8 kJ/mol.
Learn more about enthalpy, here:
https://brainly.com/question/29998512
#SPJ1