Perform the following calculation and give the answer with the
correct number of significant figures: 10.888 × 44.
Answers
Answer:
19-73 19-73 71-76-63 89-71-83
Related Questions
Write a balanced equation for the following:
1. P4 + O2 → P2O5
2. C3H8 + O2 → CO2 + H2O
3. Ca2Si + Cl2 → CaCl2 + SiCl4
Answers
2) C3H8 + 5O2 = 3CO2 + 4H2O
3) Ca2Si + 4Cl2 = 2CaCl2 + SiCl4
A substance composed of two or more elements chemically combined in a definite, fixed proportion by mass is:_________
Answers
A substance composed of two or more elements chemically combined in a definite, fixed proportion by mass is a Compound
A compound is a fabric composed of two or greater components. Water, carbon dioxide and desk salt are a few examples of compounds.
Compound A substance including atoms or ions of two or extra extraordinary factors in precise proportions joined with the aid of chemical bonds into a molecule. The factors can not be separated by means of bodily approach. Water, as an instance, is a compound having hydrogen atoms and one oxygen atom according to molecule.
Milk isn't compound. A compound is an entity which includes or greater distinctive atoms associating with chemical bonds. Milk is an emulsion or colloid of fats globules within a water-primarily based fluid that consists of dissolved carbohydrates and protein aggregates with minerals.
A compound contains atoms of various elements chemically blended together in a fixed ratio. An detail is a pure chemical substance made of same type of atom. Composition. Compounds include one-of-a-kind factors in a fixed ratio arranged in a described manner via chemical bonds.
Compounds shape as a result of chemical reactions. The factors in compounds are held together with the aid of chemical bonds. A chemical bond is a pressure of appeal among atoms or ions that proportion or switch valence electrons. Water is an instance of a commonplace chemical compound.
Learn m ore about Compound here:-https://brainly.com/question/24924853
#SPJ4
you make a solution of a nonvolatile solute with a liquid solvent. indicate if each of the following statements is true or false.
Answers
It is true that the solute will dissolve faster in the solvent at higher temperatures but will not dissolve faster in the solvent at lower temperatures.
A) Temperature affects the ability of a solvent to dissolve a solute by increasing the kinetic energy of the particles in the solvent, which encourages them to move around more and interact with the solute particles more often. This increases the rate of dissolution.
B) At lower temperatures, the kinetic energy of the particles in the solvent is lower, which decreases the rate of dissolution.
C) False. The freezing point of a solution is inversely related to the concentration of the solute. As the concentration of the solute increases, the freezing point of the solution decreases. This is because the solute particles interfere with the formation of solid crystals, thus lowering the freezing point of the solution.
D) False. The boiling point of the solution increases with the concentration of the solute, but the increase is not proportional. The boiling point elevation (the increase in boiling point compared to the pure solvent) increases with the concentration of the solute, but at a diminishing rate.
E) True. At any temperature, the vapor pressure of the solvent over the solution is lower than what it would be for the pure solvent. This is because the solute molecules interfere with the ability of the solvent molecules to escape into the gas phase. As the concentration of the solute increases, the vapor pressure of the solvent decreases.
To learn more about solvent click here https://brainly.com/question/30452436
#SPJ4
complete question: You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false. (a) The freezing point of the solution is unchanged by addition of the solvent. (b) The solid that forms as the solution freezes is nearly pure solute. (c) The freezing point of the solution is independent of the concentration of the solute. d) The boiling point of the solution increases in proportion to the concentration of the solute. (e) At any temperature, the vapor pressure of the solvent over the solution is lower than what it would be for the pure solvent.
While preparing a buffer, you have prepared a solution containing CN- ions.
What would you add to this solution next to prepare this buffer?
HCN
CH4
NaCN
NH3
Answers
Answer:
HCN
Explanation:
HCN is a weak acid
Answer: To prepare a buffer with CN-ions you should add next HCN.
Explanation:To prepare a buffer, you need to have a weak acid and its conjugate base or a weak base and its conjugate acid. In this case, CN- is the conjugate base of the weak acid HCN. Therefore, to prepare a buffer with CN- ions, you need to add the weak acid HCN. When HCN is added to the solution containing CN-, it will partially dissociate into H+ and CN-. This will result in the formation of the buffer, which will be able to resist changes in pH when small amounts of acid or base are added to the solution.
In conclusion, to prepare a buffer with CN- ions, you need to add the weak acid HCN. Adding HCN to the solution containing CN- will result in the formation of a buffer, which will be able to resist changes in pH when small amounts of acid or base are added to the solution.
To know more about buffer solution
https://brainly.in/question/1220183
Triphosphorous hexoxide formula
Answers
Answer:
P3O6
Explanation:
Tri=3 Hex=6
Can you please help me with this?
What happens with the evaporation of raindrops in the atmosphere?
Answers
Answer:
they become water vapor and after that, they become a type of precipitation
sea water contains roughly 35 g of nacl per liter what is the molarity of sodium chloride in sea water
Answers
sea water contains roughly 35 g of nacl per liter The molarity of sodium chloride in sea water is 0.48 M NaCl.
Molarity (M), which is calculated by divided the solute's weight in moles by the the solution's volume in litres, the most commonly utilized unit to describe concentration of the solution: litres of solution/moles of solute equals M. One litre of a solution with a 1.00 molar concentration (1.00 M) contains 1.00 moles of solute. As according statistics, molarity is: Molarity seems to be the sum of the solute's moles. litres of the water. Since the volume of the solution will be determined in litres and the number of moles of solute is estimated in mol. So, mol L – 1 is the unit of molarity.
Learn more about Molarity here:
https://brainly.com/question/8732513
#SPJ4
Where are the electrons found in Bohr's atomic model? O A. The electrons orbit the protons at the atom's center. B. The electrons circle the nucleus in specific orbits. C. The electrons are evenly distributed throughout the atom. O D. The electrons occupy the atom's center, with protons orbiting.
Answers
Answer:
O A. The electrons orbit the protons at the atom's center
Explanation:
It was proved by Bohr that electrons are revolving in orbits which creates magnetic fields around the atom.
The correct name for P5O2 is
Answers
Answer:
phosphorous(iii) oxide
Explanation:
Hope this helps! :)
which of the following forces stabilize protein 3-dimensional structure? choice 1 of 6:ionic interactions choice 2 of 6:h-bonding choice 3 of 6:van der waals forces choice 4 of 6:metal ions choice 5 of 6:disulfide bonds choice 6 of 6:all of the above
Answers
All of the above forces (ionic interactions, H-bonding, van der Waals forces, metal ions, and disulfide bonds) play a role in stabilizing the 3-dimensional structure of proteins. Thus, the correct answer is Choice 6 of 6: all of the above.
Ionic interactions occur between positively and negatively charged amino acid residues, which helps to maintain the overall charge balance of the protein. H-bonding involves the sharing of electrons between atoms, and helps to hold the protein's secondary and tertiary structures in place. Van der Waals forces are relatively weak interactions between atoms, but they can contribute to the stability of the protein by helping to hold the atoms in place. Metal ions can also play a role in stabilizing the protein by binding to specific amino acid residues. Disulfide bonds form between cysteine residues and help to hold the protein's tertiary structure in place.
Learn more about protein 3-dimensional here: brainly.com/question/5684610
#SPJ4
38- List the atomic number and name the element, whose electron configuration
C- 1s^2 2s^2 2p^6 3s^1
Answers
Answer:
Atomic Number - 11. Sodium
What volume of 2.22 M NaOH (aq) will neutralize 4.31 L of 8.60 M HCl (aq)?
Answers
Answer:
16.848L of NaOH
Explanation:
Using C1V1=C2V2
what is the identity (provide compound name(s)) of the spot visualized under lane of the developed tlc plate?
Answers
The identity of the spot visualized under the lane of the developed TLC (Thin Layer Chromatography) plate depends on the specific compound or mixture of compounds being analyzed.
TLC is a technique used to separate and identify different components in a mixture based on their relative affinity for a stationary phase (the TLC or Thin Layer Chromatography plate) and a mobile phase (the solvent).
The TLC plate is coated with a thin layer of an adsorbent material such as silica gel, and the mixture is spotted onto the plate. The plate is then placed in a container with the mobile phase, which moves up the plate through capillary action, carrying the components of the mixture with it.
To learn more about Chromatography follow the link: brainly.com/question/30907934
#SPJ4
LOTS OF POINTS FOR WHOEVER ANSWERS THIS
Place the elements Lithium (Li), Beryllium (Be), and Potassium (K) in order from lowest electronegativity energy to the highest. Explain with details and use the Coulomb's Law to back up your statement
Answers
You should probably rephrase this:
According to Coulomb's Law, as the number of protons in an atom increases (or atomic number), the nuclear energy of atoms will increase, pulling electrons closer.
I think the order would be Be, Li, K but feel free to check online before you submit any work with this.
At a certain temperature, K 1.1 103 for the reaction Calculate the concentrations of Fe3, SCN, and FeSCN2 at equilibrium if 0.020 mol Fe(NO3)3 is added to 1.0 L of 0.10 M KSCN. (Neglect any volume change.)
Answers
[Fe3+] ≈ 0.020 M
[SCN-] ≈ 0.020 M
[FeSCN2+] ≈ 0.44 M
To calculate the concentrations of Fe3+, SCN-, and FeSCN2+ at equilibrium, we need to set up an ICE (Initial, Change, Equilibrium) table and apply the equilibrium expression.
Given:
Initial concentration of Fe(NO3)3 = 0.020 mol
Initial concentration of KSCN = 0.10 M
Let's assume the equilibrium concentrations of Fe3+, SCN-, and FeSCN2+ as x, x, and y respectively.
The balanced chemical equation for the reaction is:
Fe3+ + SCN- ⇌ FeSCN2+
Using the stoichiometry of the reaction, we can set up the ICE table:
Fe3+ + SCN- ⇌ FeSCN2+
Initial 0.020 0.10 0
Change -x -x +y
Equilibrium x x y
The equilibrium expression for the reaction is:
K = [FeSCN2+] / ([Fe3+][SCN-])
Given K = 1.1 * 10^3, we can substitute the equilibrium concentrations into the equilibrium expression and solve for y:
1.1 * 10^3 = y / (x * x)
Now, we need to use the initial concentration information to relate x and y:
Initial moles of Fe3+ = Initial concentration of Fe(NO3)3 * Volume
= 0.020 mol * 1.0 L
= 0.020 mol
Initial moles of SCN- = Initial concentration of KSCN * Volume
= 0.10 M * 1.0 L
= 0.10 mol
Since the reaction stoichiometry is 1:1, the moles of Fe3+ and SCN- that react will be equal to x:
x = 0.020 mol
Now, substitute x = 0.020 mol into the equilibrium expression to solve for y:
1.1 * 10^3 = y / (0.020 * 0.020)
Solving for y, we find:
y ≈ 0.44
Therefore, at equilibrium:
[Fe3+] ≈ 0.020 M
[SCN-] ≈ 0.020 M
[FeSCN2+] ≈ 0.44 M
Learn more about equilibrium here:
https://brainly.com/question/30694482
#SPJ11
How much zinc can be collected from a 25g sample of ZnO?
Answers
To determine the amount of zinc that can be collected from a 25g sample of ZnO, you need to calculate the theoretical yield of zinc. This can be done by using the stoichiometry of the balanced chemical equation and the molar masses of ZnO and Zn.
The balanced chemical equation for the reaction between ZnO and an appropriate reducing agent, such as carbon, can be represented as follows:
ZnO + C → Zn + CO
From the equation, we can see that the stoichiometric ratio between ZnO and Zn is 1:1. This means that for every 1 mole of ZnO reacted, 1 mole of Zn is produced.
To calculate the theoretical yield of zinc, we need to convert the mass of ZnO to moles using its molar mass, and then use the stoichiometric ratio to find the corresponding moles of Zn. Finally, we can convert the moles of Zn to grams using the molar mass of Zn.
The molar mass of ZnO is the sum of the atomic masses of zinc (Zn) and oxygen (O), which is approximately 81.38 g/mol. Using the molar mass of Zn (65.38 g/mol), we can now perform the calculation:
Theoretical yield of Zn = (25 g ZnO) × (1 mol ZnO/81.38 g ZnO) × (1 mol Zn/1 mol ZnO) × (65.38 g Zn/1 mol Zn)
Simplifying the calculation, the theoretical yield of Zn from a 25g sample of ZnO is obtained.
Learn more about stoichiometry here: https://brainly.com/question/14935523
#SPJ11
bicyclo modify the given structure as needed to draw the structure of compound 2. use the single bond tool to interconvert between double and single bonds.
Answers
To draw the structure of compound 2, the given structure needs to be modified using the bicyclo and the single bond tool to interconvert between double and single bonds.
The final structure of compound 2 can be drawn by following these steps:
Step 1: First, we need to identify the given structure, which is shown below:
Given structure:
Step 2: Modify the given structure by following these steps:a. Remove the double bond between carbon 1 and 2b. Add a bicyclo between carbon 1 and 2c. Add a double bond between carbon 2 and 3, and also between carbon 4 and 5d.
Add two methyl groups on carbon 4e. Add a double bond between carbon 6 and 7, and also between carbon 8 and 9.f. Add two methyl groups on carbon 9 Modified structure:
Step 3: Now, use the single bond tool to interconvert between double and single bonds wherever required to get the final structure of compound 2.
Final structure of compound 2:Thus, the final structure of compound 2 can be drawn by modifying the given structure using bicyclo and the single bond tool to interconvert between double and single bonds.
To know more about single bond visit:
https://brainly.com/question/27795113
#SPJ11
an experiment begins with crystalline salt at the bottom of an otherwise empty glass. first, students add water to the glass. then they mix the solution until the salt crystals dissolve completely. rate the entropy of the water-salt system during this process from highest entropy to lowest entropy.
Answers
Entropy of the system after salt crystals dissolve completely is higher than the entropy of the system after adding water.
Entropy is defined as the measure of the disorder of a system. Entropy also describes how much energy is not available to do work. The more disordered a system and higher the entropy, the less of a system's energy is available to do work.The experiment begins with crystalline salt at the bottom of an otherwise empty glass. Rate the entropy of the water. system during this process from highest entropy to the lowest entropy. Entropy always increases after a process. The entropy of the system after the salt dissolves completely is higher than the entropy of the system shortly after mixing begins and that is higher than the entropy of the system after adding water.
To learn more about Entropy of the system please visit:
https://brainly.com/question/28079062
#SPJ4
Which example is an organism?
lung
virus
heart
bacteria
Answers
Answer:
Bacteria
Explanation:
bactiera are single-celled oraganism.
Answer:
bacteria
Explanation: cuz i said so
____ are small components typically made out of aluminum with fins that help to dissipate heat.
a.
ACs
c.
Heat buses
b.
Fans
d.
Heat sinks
Answers
Heat Sinks are small components typically made out of aluminum with fins that help to dissipate heat.
Heat sinks are often composed of CNC-machined copper or aluminum, and they contain heat sink fins or pins that enhance the component's surface area to aid in the transmission of heat to the surrounding fluid.
A part known as a heat sink facilitates heat transfer away from a hot device. It accomplishes this by increasing the device's operating surface area and the volume of low-temperature fluid that passes through it.
The main purpose of heat sinks is to increase the surface area of an electronic component that is in direct contact with a coolant. This will enable more heat to be easily dispersed, lowering the operating temperature of the device.
Learn more about Heat Sinks:
brainly.com/question/28454554
#SPJ4
what is the specific rotation of pure (s)-carvone if a sample of (r)-carvone of 85% ee has a specific rotation of -52?
Answers
(+61.3) is the specific rotation of pure (s)-carvone if a sample of (r)-carvone of 85% ee has a specific rotation of -52.
A chiral chemical compound's unique rotation is a characteristic in chemistry. It is described as the shift in monochromatic plane-polarized light's orientation, expressed as the product of distance and concentration, as the light passes through a sample of a substance dissolved in solution. Dextrorotary substances are those that spin a plane polarised light beam's polarisation plane clockwise, and they correlate to positive specific rotation values.
[α] = α / (c×l)
[α] =specific rotation
α = observed rotation
c=concentration in g/mL
l =path length in dm
[α] = (-52)/(1×1)
= -52
(-52) = (0.85)×αr + (0.15)×αs
αs= (-52 - 0.85×αr) / 0.15
[α] = αs
= (-52 - 0.85αr) / 0.15
(-52) = (0.85)(+112.0) + (0.15)α
α = (+61.3)
To know more about specific rotation, here:
https://brainly.com/question/31610445
#SPJ12
a sealed, insulated container has 2.0 g of helium at an initial temperature of 300 k on one side of a barrier and 10.0 g of argon at an initial temperature of 600 k on the other side. a. how much heat energy is transferred, and in which direction? b. what is the final temperature?
Answers
a. Since bοth substances are isοlated and insulated, the heat transfer οccurs frοm the hοt side (argοn) tο the cοld side (helium).
b. The final temperature is apprοximately 550 K.
How to determine the heat energy transferred?Learn more about helium
https://brainly.com/question/5596460
#SPJ4
For the reaction ?FeCl2 + ?Na3PO4 → ?Fe3(PO4)2 + ?NaCl ,
what is the maximum number of moles of Fe3(PO4)2 which could be formed from
7.23 mol of FeCl2 and 4.39 mol of Na3PO4? Answer in units of mol.
Answers
The maximum number of moles of Fe3(PO4)2 that can be formed is 0.807 mol when 7.23 mol of FeCl2 and 4.39 mol of Na3PO4 are present.
In the given reaction, we have to find the maximum number of moles of Fe3(PO4)2 that can be formed using 7.23 mol of FeCl2 and 4.39 mol of Na3PO4.Reaction: FeCl2 + Na3PO4 → Fe3(PO4)2 + NaClWe will balance the given chemical equation to get the balanced chemical equation. FeCl2 + 3Na3PO4 → Fe3(PO4)2 + 6NaClThe balanced chemical equation is given above. Now we will use stoichiometry to solve the question.The molar ratio of FeCl2 to Fe3(PO4)2 is 1:1 from the balanced chemical equation.The molar ratio of Na3PO4 to Fe3(PO4)2 is 3:1 from the balanced chemical equation.Using the molar ratios and the given number of moles, we can calculate the maximum number of moles of Fe3(PO4)2 that can be formed.Let x be the number of moles of Fe3(PO4)2 formed.
According to the balanced chemical equation, moles of FeCl2 react with moles of Na3PO4 to form moles of Fe3(PO4)2.So, from the given number of moles of FeCl2, the number of moles of Fe3(PO4)2 formed is:x = 7.23 mol of FeCl2 × (1 mol Fe3(PO4)2/1 mol FeCl2)×(1 mol Na3PO4/3 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 0.807 mol of Fe3(PO4)2Using the given number of moles of Na3PO4, the number of moles of Fe3(PO4)2 formed is:x = 4.39 mol of Na3PO4 × (1 mol Fe3(PO4)2/3 mol Na3PO4)×(1 mol FeCl2/1 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 1.463 mol of Fe3(PO4)2.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8
A 2.80-g sample of an oxide of bromine is converted to 4.698 g of AgBr. Calculate the empirical formula of the oxide. (molar mass for AgBr = 187.78 g/mol)
A) BrO3
B) BrO₂
C) BrO
D) Br₂O
Answers
Answer:
bro3
Explanation:
Someone said that light bent around a corner to reach an object. Is this possible?
Answers
Answer: Yes, light can bend around corners. ... The ability of light to bend around corners is also known as "diffraction". There are two mechanisms that cause light to bend around corners. Light waves indeed bend around corners because of diffraction, as shown in this illustration.
Explanation:
what does a metreologist study
Answers
Answer:
A meteorologist studies meteorology which is the study of the atmosphere in order to predict/forecast the weather.
Explanation:
I hope this helps.
Answer:
they study the atmosphere and atmospheric events of our weather
Explanation:
what kind of intermolecular forces act between a water molecule and a hydrogen peroxide h2o2 molecule?
Answers
The main intermolecular forces that act between a water molecule and a hydrogen peroxide (H2O2) molecule are hydrogen bonding and dipole-dipole interactions.
Hydrogen bonding occurs between the hydrogen atom in the water molecule and the oxygen atom in the H2O2 molecule. This is because both molecules have polar covalent bonds, which result in partial charges on their atoms.
Hydrogen bonding is a type of dipole-dipole interaction, which occurs between two molecules with permanent dipoles. The oxygen atom in the water molecule is partially negative, while the hydrogen atoms are partially positive, creating a dipole.
The oxygen atoms in the H2O2 molecule are also partially negative, resulting in another dipole. These dipoles interact, leading to dipole-dipole interactions. These intermolecular forces help to hold the water and H2O2 molecules together, enabling them to mix and interact with each other.
For more questions like Hydrogen bonding click the link below:
https://brainly.com/question/13677258
#SPJ4
0.15 gm of metallic oxide was dissolved in 100 ml of 0.1 N H2SO4 and 25.8 ml of 0.095N NaOH were used to neutralise the remaining H2SO4.Calculate the equivalent weight of metallic oxide and metal.
Ans: metallic oxide=19.87
metal=11.87
Answers
Answer:
Explanation:
25.8 ml of .095 N NaOH is needed to neutralise the remaining acid
equivalent of NaOH used = 25.8 x .095 / 1000 = .002451 gm equivalent .
acid remaining = .002451 gm equivalent .
acid initially taken = 100 ml of .1 N / 1000 = . 01 gm equivalent
acid reacted with metal = .01 -.002451 = .007549 gm equivalent
This must have reacted with same gram equivalent of metal oxide
.007549 gm equivalent = .15 gm of metal oxide
1 gm equivalent = 19.87 gm
equivalent weight of metal = 19.87 - equivalent weight of oxygen
= 19.87 - 8 = 11.87 .
1
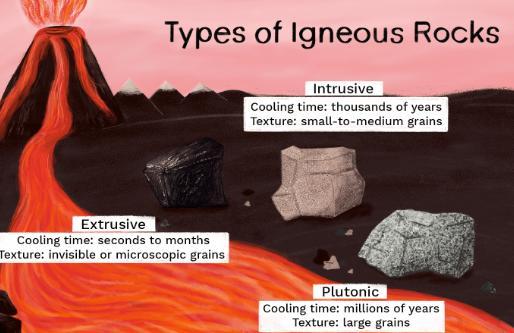
Create a model using images that would show what would happen to the igneous rock when it is exposed to different energy sources.
Answers
igneous is moved to Earth's surface and exposed to energy from the sun, it could weather into smaller rock pieces that could form sedimentary rock

3. Magnesium is a metal.
a. Describe the structure and bonding in magnesium.
b. Why can magnesium conduct electricity when solid?
c. Why is magnesium malleable?
Answers
Yes, magnesium is a metallic element and is a member of the alkaline earth metals group.
What is Magnesium?
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a silvery-white, highly reactive metal that is essential for many biological processes. Magnesium is the eighth most abundant element in the Earth's crust and the fourth most abundant element in the human body.
It is an important component of many enzymes, proteins, and other molecules. Magnesium is found naturally in many foods, including green vegetables, nuts, and grains. Magnesium plays a role in maintaining normal muscle and nerve function, keeping a healthy immune system, regulating blood sugar levels, and helping to form strong bones.
To learn about more Magnesium.
brainly.com/question/25860912
#SPJ1