One of the main goals of environmental science is to help humans use the environment in a way that is economically profitable.
true or false
Answers
Answer:
true this is correct its your econimically
Related Questions
The haber process can be used to produce ammonia (nh3) from hydrogen gas (h2) and nitrogen gas (n2). the balanced equation for this process is shown below. 3h2 n2 right arrow. 2nh3 the molar mass of nh3 is 17.03 g/mol. the molar mass of h2 is 2.0158 g/mol. in a particular reaction, 0.575 g of nh3 forms. what is the mass, in grams, of h2 that must have reacted, to the correct number of significant figures? 0.1 grams 0.102 grams 0.10209 grams 0.1021 grams
Answers
The mass of hydrogen reacted with nitrogen to give ammonia is 0.1 grams.
How we calculate the mass from moles?
Mass of any substance will be calculated by using the moles as:
n = W/M, where
W = required mass
M = molar mass
Given chemical reaction is:
3H₂ + N₂ → 2NH₃
Moles of 0.575 g of NH₃ will be calculated as:
n = 0.575g / 17.03g/mol = 0.033 moles
From the stoichiometry of the reaction, it is clear that:
2 moles of NH₃ = produced by 3 moles of H₂
0.033 moles of NH₃ = produced by 3/2×0.033=0.0495 moles of H₂
Now we calculate the mass of hydrogen from the given moles and molar mass as:
W = (0.0495mol)(2.0158g/mol) = 0.09 grams = 0.10 grams
Hence, option (a) is correct i.e. 0.1 grams.
To know more about moles, visit the below link:
https://brainly.com/question/15374113
If greenhouse gases in the atmosphere act like a blanket around the Earth, how is this "blanket” being changed by human activities?
It is getting thinner and keeping the Earth cooler.
It is getting thicker and keeping the Earth cooler.
It is getting thicker and keeping the Earth warmer.
It is getting thinner and keeping the Earth warmer.
Answers
Answer:
It is getting thicker and keeping the Earth warmer.
Answer:
it is getting thinner and keeping the earth warmer
a solution containing a mixture of metal cations was treated as outlined. dilute hcl hcl was added and no precipitate formed. h2s h 2 s was bubbled through the acidic solution. a precipitate formed and was filtered off. the ph was raised to about 9 9 and h2s h 2 s was again bubbled through the solution. a precipitate formed and was filtered off. finally, sodium carbonate was added to the filtered solution and no precipitate formed. what can be said about the presence of each of these groups of cations in the original solution?
Answers
The tests suggest that the original solution contains Group 2 cations (Zn²⁺, Cd²⁺, Pb²⁺), Group 3 cations (Fe³⁺), and possibly Group 4 cations (Ni²⁺, Co²⁺), but does not contain Group 1 cations, Group 2 cations (Mg²⁺), Group 3 cations (Al³⁺), Group 4 cations (Mn²⁺), or Group 5 cations (Cu²⁺).
No precipitate formed when dilute HCl was added, indicating the absence of Group 2 cations (Ca²⁺, Sr²⁺, Ba²⁺) and Group 3 cations (Al³⁺). When H₂S was bubbled through the acidic solution, a precipitate formed and was filtered off. This indicates the presence of Group 2 cations (Zn²⁺, Cd²⁺, Pb²⁺), Group 3 cations (Fe³⁺, Al³⁺), and Group 4 cations (Ni²⁺, Co²⁺, Mn²⁺).
The pH was raised to about 9 and H₂S was bubbled through the solution again. Another precipitate formed and was filtered off. This indicates the presence of Group 4 cations (Ni²⁺, Co²⁺) and Group 5 cations (Cu²⁺). Finally, sodium carbonate was added to the filtered solution and no precipitate formed, indicating the absence of Group 1 cations (Li⁺, Na⁺, K⁺), Group 2 cations (Mg²⁺, Ca²⁺, Sr²⁺, Ba²⁺), Group 3 cations (Al³⁺), Group 4 cations (Ni²⁺, Co²⁺, Mn²⁺), and Group 5 cations (Cu²⁺).
To know more about precipitate, here
https://brainly.com/question/14097253
#SPJ4
We learned in class that ... is from the foxglove plant and that ... causes strong hallucinations.
taxol
peyote
turmeric
digitalis
saffron
CBD
Answers
We learned in class that digitalis is from the foxglove plant and that it causes strong hallucinations. Digitalis is commonly used to treat heart conditions but can also be dangerous when taken in large amounts.
On the other hand, peyote is a cactus that contains the hallucinogenic compound mescaline. It has been traditionally used in Native American religious ceremonies. Turmeric is a spice commonly used in Indian cuisine that has anti-inflammatory properties. Taxol, derived from the Pacific yew tree, is used in chemotherapy to treat cancer. Saffron is a spice derived from the crocus flower and has been studied for its potential anti-depressant effects. CBD, derived from the hemp plant, is a non-psychoactive compound that has been studied for its potential therapeutic uses.
It seems you are discussing the properties of different plants. Digitalis, derived from the foxglove plant, is known for its medicinal properties in treating heart conditions. On the other hand, peyote, a type of cactus, contains psychoactive compounds that cause strong hallucinations. Taxol is a chemotherapy drug extracted from the yew tree, while turmeric and saffron are popular spices with numerous health benefits. Lastly, CBD is a non-psychoactive compound found in cannabis, often used for its therapeutic effects.
To learn more about digitalis visit;
https://brainly.com/question/4172320
#SPJ11
Select the curve that is produced by adding hydrochloric acid to 25 cm3 of sodium hydroxide.A,B,C or D

Answers
B
The sodium hydroxide (NaOH) solution is a basic solution, so the pH of that solution should be close to 14
then when adding hydrochloric acid (HCl) we start to neutralice the solution, meaning the pH must sift slowly to lower pH.
Assuming both solutions have similar concentration the pH shall shift form basic (above 7) to acid pH (below 7). Until now both B and D images agreed with the explanation given. To chose between them we need to remember that HCl is a very strong acid, which means that in solution will get to very acid solutions (very low pH values) which leaves only B as possible answer
How many grams are in 21.7 mol of H2O?
Answers
Answer:
390.6 gram
Explanation:
Mass in gram = no of moles × molar mass
Mass in gram = 21.7×18
= 390.6 gram
Answer:
390.931576 grams
the table gives the composition of three particles
(a) what is the evidence in the table for each of the following?
(i)Particle A is an atom
(ii) A,B and C are all particles of the same element.
(iii) Particles A and C are isotopes of the same element.
(b) (i) What is the electronic structure of particle A?
(ii) Is element A , a metal or non-metal? Give a reason for your choice

Answers
The evidence in the table for each of the following atoms of an element A, B, and C is that A,B, and C are all particles of the same element; option ii.
What are elements?Elements are substances that are composed entirely of the same atoms and which cannot be split into simpler units by an ordinary chemical process.
Atoms of the same element have the same number of protons or the same atomic number.
However, atoms of the same element may have different numbers of neutrons, and these atoms of the element are called isotopes.
Learn more about elements at: https://brainly.com/question/6258301
#SPJ1
State the number of electrons, protons, and neutrons (in order) for N.
Answers
Nitrogen has 7 electrons, 7 protons, and 7 neutrons in its most common isotope.
What are the number of electrons, protons, and neutrons in nitrogen?
Nitrogen (N) is a chemical element with an atomic number of 7, which means it has 7 protons in its nucleus. Since nitrogen is a neutral element, it also has 7 electrons orbiting around the nucleus, balancing out the positive charge of the protons. The most common isotope of nitrogen has 7 neutrons in its nucleus, giving it a mass number of 14 (since the mass number is equal to the sum of protons and neutrons in the nucleus).
However, there are other isotopes of nitrogen that can have different numbers of neutrons. The presence or absence of neutrons in an atom's nucleus can affect its stability and reactivity, making isotopes important in various scientific and industrial applications.
To learn more about isotope, visit: https://brainly.com/question/14220416
#SPJ4
describe the relationship between insulin resistance and the three common clinical hallmarks of T2DM: elevated fasting blood glucose, sustained OGTT, and elevated blood fatty acid levels.
Answers
Insulin resistance is a key factor in the development of T2DM, leading to elevated fasting blood glucose, sustained OGTT, and elevated blood fatty acid levels.
Insulin resistance occurs when the body's cells become less responsive to insulin, which is needed to move glucose from the bloodstream into cells for energy. As a result, glucose accumulates in the bloodstream, leading to elevated fasting blood glucose levels. Sustained OGTT (oral glucose tolerance test) occurs when the body is unable to properly regulate blood glucose levels after consuming a high-carbohydrate meal, which is a hallmark of T2DM.
Insulin resistance also causes the body to release more fatty acids into the bloodstream, leading to elevated blood fatty acid levels. This can further exacerbate insulin resistance by interfering with insulin signaling pathways. Therefore, addressing insulin resistance is key to managing T2DM and preventing its complications.
Learn more about insulin here:
https://brainly.com/question/28209571
#SPJ11
City A in the Southern Hemisphere and City B in the Northern Hemisphere are located at the same latitude. Which statement is likely true about these
cities?
City B has the larger annual temperature range.
Both cities should have nearly identical winter temperatures.
City A has the larger annual temperature range.
Both cities likely have the same annual temperature range.
Answers
Answer:
City B has the larger annual temperature range
Explanation:
This is correct option because generally the northern side of the equator is high in temperature than the southern hemisphere part.
Since the southern side of the equator or Southern Hemisphere, where city A resides will generally have higher altitude or rise, so this creates higher average temperature.
How many moles of O2 are needed to produce 30 g of Fez0s?
Answers
The number of mole of oxygen gas, O₂ needed to produced 30 grams of Fe₂O₃ is 0.282 mole
How do i determine the number of mole of O₂ needed?First, we shall obtain the mole of 30 grams of Fe₂O₃. This is shown below:
Mass of Fe₂O₃ = 30 grams Molar mass of Fe₂O₃ = 159.69 g/mol Mole of Fe₂O₃ =?Mole = mass / molar mass
Mole of Fe₂O₃ = 30 / 159.69
Mole of Fe₂O₃ = 0.188 mole
Finally, we shall obtain the number of mole of O₂ needed. This is shown below:
4Fe + 3O₂ -> 2Fe₂O₃
From the balanced equation above,
2 moles of Fe₂O₃ were obtained from 3 moles of O₂.
Therefore,
0.188 mole of Fe₂O₃ will be obtain from = (0.188 × 3) / 2 = 0.282 mole of O₂
Thus, we can conclude that number of mole O₂ needed is 0.282 mole
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
Complete question:
How many moles of O₂ are needed to produce 30 g of Fe₂O₃?
CHEMISTRY 50 POINTS!
Which of the following explains the VSEPR geometry of a water molecule?
A) It is tetrahedral because there are four bonded pairs around oxygen.
B) It is bent because there are four bonded pairs around oxygen.
C) It is tetrahedral because there are two bonded pairs and two lone pairs around oxygen.
D) It is bent because there are two bonded pairs and two lone pairs around oxygen.
Answers
Answer: D
Explanation:
Water is comprised of 2 Hydrogen atoms and 1 Oxygen atom. This gives us a total of 8 valence electrons to form bonds with.
Because Hydrogen is in first row of elements it can only hold a total of two electrons. This means each Hydrogen holds 2 electrons.
But that means there are four more electrons. VSEPR theory is basically how electron repulsion causes atoms to arrange in different ways.
There will be four electrons left on the oxygen because they can not go anywhere else. This will cause the hydrogen molecules to move away from the lone electrons and cause a bent geometry rather than a straight-line geometry.
A 4.60 L container with a moveable
piston has a pressure of 845 mm Hg.
What is the pressure when the
container is expanded to 10.6 L?
P = [?] mm Hg
Answers
P2 = 366 mm Hg
Step-by-step explanation:
We can use the ideal gas law to solve this problem:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature.
Assuming that the number of moles of gas and the temperature remain constant, we can write:
P1V1 = P2V2
where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Substituting the given values into the equation, we get:
P1 = 845 mm Hg
V1 = 4.60 L
V2 = 10.6 L
P2 = P1 * V1 / V2
P2 = 845 mm Hg * 4.60 L / 10.6 L
P2 = 366 mm Hg
Therefore, the pressure when the container is expanded to 10.6 L is 366 mm Hg.
During the procedure, several wases with cold methanol were done. why was it important to use cold methanol? (In nitration of methyl benzoate)
Answers
The use of cold methanol is important because it is used to recrystallize or solidify the product.
Since, ice bath is also used in nitration of methyl benzoate because in this reaction the concentrated acid like sulfuric acid is used with water and it is very exothermic.
The formation pure solid from the impure solid compound by mixing with hot solvent and form saturated solution. Now, this solution as cools and pure crystal grows when solubility of the compound decreases. This whole process is called re-crystallization.
learn about re-crystallization
https://brainly.com/question/14918321
#SPJ4
,P₁
V₁=X
1. A 3.0 L container of argon has a pressure of 3.5 atm. What volume would be necessary
to increase the pressure to 557.15 kPa?
182
Answers
1.64 L volume would be necessary to increase the pressure to 557.15 kPa.
What is pressure?In the physical sciences, pressure is defined as the perpendicular force per unit area or the stress at a point within a confined fluid. A 42-pound box with a bottom area of 84 square inches will press down on a surface with a pressure equal to the force divided by the area over which it is applied, or half a pound per square inch.
Atmospheric pressure, which at sea level is roughly 15 pounds per square inch, is the weight of the atmosphere pressing down on each unit area of the Earth's surface. One pascal is equal to one newton per square metre when expressed in SI units, which is how pressure is calculated. Nearly 100,000 pascals of atmospheric pressure are present.
We can obtain the answer using Boyle's Law
\(\frac{P_1 \times V_1}{T_1} = \frac{P_2 \times V_2}{T_2}\)
As the temperature is constant,
\(P_1 \times V_1}= P_2 \times V_2}\)
We have given that
P₁ = 3.5 atm
= 303.975 kPa (One atm equals 101.325 kPa)
V₁ = 3.0 L
P₂ = 557.15 kPa
V₂ = To find
Lets substitute the values
⇒ 303.975 kPa × 3.0 L = 557.15 kPa × V₂
⇒ V₂ = (303.975 kPa × 3.0 L) / 557.15 kPa
⇒ V₂ = 1.64 L
Learn more about pressure
https://brainly.com/question/945436
#SPJ9
The number of atoms in 5 moles of Ag?
Answers
Answer:
3x10^24 atoms to 1 sig fig
Explanation:
By definition, there are 6.022x10^23 atoms in a mole.
Multiply this by the 5 moles of silver to find the atoms of silver.
(5 moles)*(6.022x10^23 atoms/mole) = 30.110x10^23 atoms
3x10^24 atoms to 1 sig fig
A flask contains 2.00 moles of nitrogen and 2.00 moles of helium. How many grams of argon must be pumped into the flask in order to make the partial pressure of argon twice that of helium
Answers
The amount of argon will be zero gram.
Assuming ideal gas behavior, we can use the following formula to calculate the partial pressure of a gas in a mixture:
Partial pressure = (moles of gas / total moles of gas) x total pressure
Calculating the total moles of gas in the flask:
Total moles of gas = moles of nitrogen + moles of helium = 2.00 + 2.00 = 4.00 moles
To calculate the partial pressure of helium in the flask, since we want the partial pressure of argon to be twice that of helium:
Partial pressure of helium = (moles of helium / total moles of gas) x total pressure
= (2.00 / 4.00) x total pressure = 0.5 x total pressure
To make the partial pressure of argon twice that of helium, we need to add enough argon to the flask so that its partial pressure is equal to:
2 x partial pressure of helium = 2 x 0.5 x total pressure = total pressure
Therefore, the mole fraction of argon in the flask after adding the desired amount of argon will be:
Mole fraction of argon = (partial pressure of argon / total pressure)
= 1 / 1 = 1
This means that the moles of argon need to add to the flask is:
Moles of argon = mole fraction of argon x total moles of gas - moles of nitrogen - moles of helium
= 1 x 4.00 - 2.00 - 2.00
= 0.00 moles
Therefore, the amount of argon will be zero gram.
To know more about moles
https://brainly.com/question/31597231
#SPJ4
Wath is the effect of the tempreature on the kinetic energy of constituent particles of:
(a) solid
(b) liquid
(c)gas
Answers
The impact of temperature on the kinetic energy of the component particles can be seen. The kinetic energy of particles in solids and liquids causes increasing vibration or random motion.
As temperature rises, gas particles in gas travel more quickly and clash more frequently. The effect of temperature on the kinetic energy of constituent particles can be explained as follows:
(a) Solid: As the temperature of a solid increases, the kinetic energy of its constituent particles also increases. This is because the increase in temperature leads to the particles vibrating with more energy and at a higher frequency.
The particles in a solid are closely packed together and have limited freedom of movement. Therefore, the increase in kinetic energy primarily manifests as increased vibration of the particles within their fixed positions.
(b) Liquid: Similar to solids, an increase in temperature also increases the kinetic energy of particles in a liquid. However, since the particles in a liquid have more freedom of movement compared to solids, the increase in kinetic energy is reflected in the increased random motion of the particles.
As the temperature rises, the liquid particles move faster and collide more frequently with each other, leading to increased overall kinetic energy.
(c) Gas: In a gas, the particles have the highest degree of freedom of movement. As the temperature of a gas increases, the kinetic energy of its particles also increases.
This increase in kinetic energy results in higher average speeds of gas particles and more frequent collisions. The gas particles move rapidly and randomly, filling the entire volume of the container they are in.
To know more about kinetic energy refer to this:
https://brainly.com/question/999862
#SPJ11
Từ hóa trị của Cl trong hợp chất HCl, hãy lập công thức hóa học của hai hợp chất do kim loại K, Ca liên kết với Cl
Answers
Answer:
kcl,cacl2
Explanation:
g looking down the ca-cb bond in the molecule below (your head must be oriented at the top of the screen), which newman-projection would be correct?
Answers
Answer:he correct Newman projection would be:
O
/ \
C C
\ /
H
Explanation:
Answer:
O
/ \
C C
\ /
H
Explanation:
why is it important to use an appropriate instrument in measuring the distance of an object?
A.it gives precise and exact measurment
B.The measurements vary
C.Helps people process data accurately
D.Both A and C
nonsense:report
not nonsense:brainly and like
pls answer it properly
it is from science
Answers
It is important to use an appropriate instrument in measuring the distance of an object because it gives precise and exact measurements and also, it ensures that the measurement taken is accurate, consistent and reliable. (option A and C)
This is important because it helps people process data accurately. If measurements are not precise or exact, it can lead to errors in data analysis, research findings, and decision-making. Both A and C are correct as an appropriate instrument ensures that the data collected is precise, which leads to more accurate data analysis and decision-making. Hence, using an appropriate instrument is crucial to obtain accurate and reliable data. (options A and C)
More on instruments: https://brainly.com/question/9949022
#SPJ11
Calculate the heat released or absorbed. Using Q = m * Cp * TCp = 4.186 J/gK
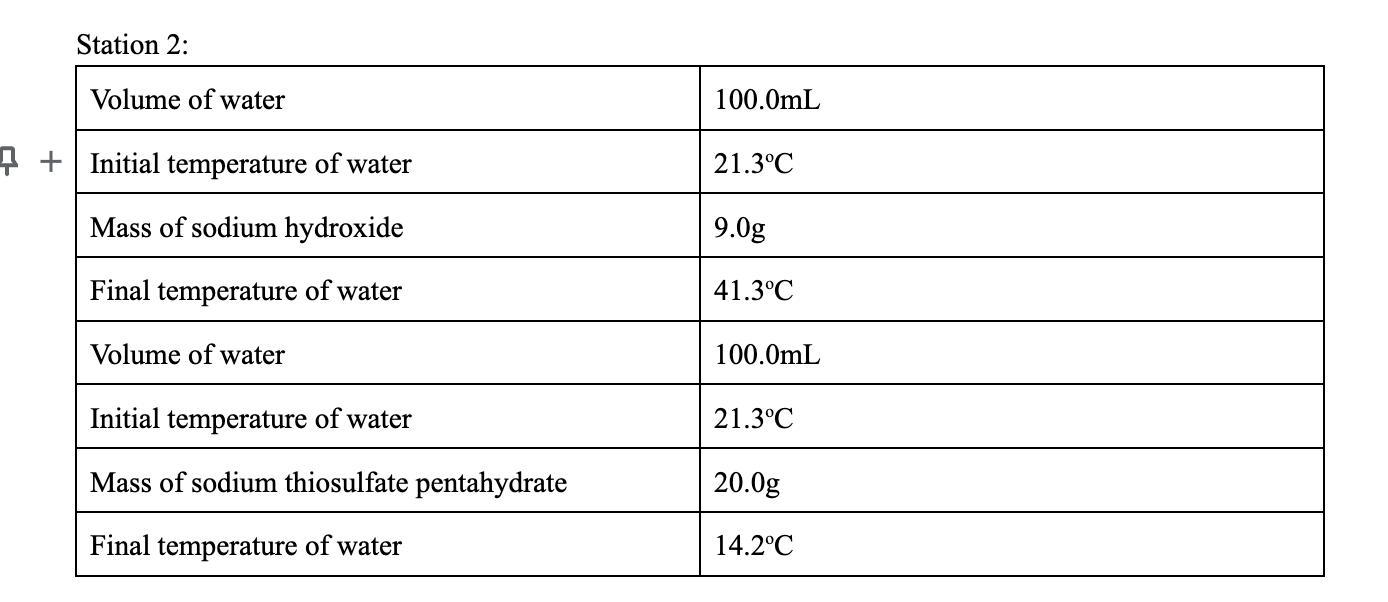
Answers
Explanation:
a) Sodium Hydroxide:
Volume of water = 100.0 mL
Initial temperature of water = 21.3 °C
Mass of NaOH = 9.0 g
Final temperature of water = 41.3 °C
So we had a 100.0 mL sample of water at 21.3 °C and after we added 9.0 g of NaOH the temperature increased to 41.3 °C. Let's find the heat that the water absorbed using the formula:
Q = m * Cp * ΔT
Where m is the mass, Cp is the specific heat of water (Cp = 4.186 J/g°C) and ΔT is the temperature change.
First we had 100.0 mL of water or 100.0 g (if we consider that the density of water is 1.0 g/mL) and we added 9.0 g of NaOH. Let's find the total mass.
m = total mass = mass of water + mass of NaOH
m = 100.0 mL * 1.0 g/mL + 9.0 g
m = 109.0 g
We can also find the change in temperature.
ΔT = Tfinal - Tinitial = 41.3 °C - 21.3 °C
ΔT = 20.0 °C
The dissolution of the NaOH is an exothermic process. This reaction will release heat that will be absorbed by its surroundings and the temperature of the water will increase.
Finally we can calculate the heat that was absorbed.
Qw = m * Cp * ΔT
Qw = 109 g * 4.186 J/(g°C) * 20.0 °C
Qw = 9125 J
So the water absorbed 9125 J and the reaction released -9125 J.
b) Sodium thiosulfate pentahydrate:
Volume of water = 100.0 mL
Initial temperature of water = 21.3 °C
Mass of sodium thiosulfate pentahydrate = 20.0 g
Final temperature of water = 14.2 °C
The dissolution of the sodium thiosulfate pentahydrate is an endothermic process in this case. The dissolution will absorb energy from its surroundings and the temperature of the water will decrease.
m = total mass = mass of water + mass of thiosulfate
m = 100.0 mL * 1.0 g/mL + 20.0 g
m = 120.0 g
ΔT = Tfinal - Tinitial = 14.2 °C - 21.3 °C
ΔT = -7.1 °C
Qw = m * Cp * ΔT
Qw = 120 g * 4.186 J/(g°C) * (-7.1 °C)
Qw = -3566 J
So the water released -3566 J and the dissolution process absorbed 3566 J.
Answer:
a) The water absorbed 9125 J and the dissolution process released -9125 J.
b) The water released -3566 J and the dissolution process absorbed 3566 J.
Velocity has both speed and direction, so it is a what
Answers
Answer: Velocity is a mathematical vector. That means it has both a magnitude (speed) and a direction. Scientists need to consider an object’s velocity to calculate its momentum — its velocity multiplied by its mass. Scientists use measures of velocity in everything from figuring out how much rain is falling to sending a rocket to space.
Explanation: Hope this help:)
which sorting algorithm has the following time complexity profile? best case: o(n log n)
Answers
Quick Sort is the sorting algorithm that has the following time complexity profile i.e., best case: o(n log n).
A Divide and Conquer method is Quick Sort. It chooses an element to act as a fulcrum and divides the supplied array around it. There are numerous variations of Quick Sort that select pivot in various methods.
1)As a rule, choose the first component as the center.
2)Always choose the final component as the center
3)Choose a random number as a pivot element.
4)Decide on median as your center.
Partitioning is Quick Sort's primary operation. The partition procedure works best when it always chooses the middle element as the pivot.
Here is more information about Quick Sort: brainly.com/question/13155236
#SPJ4
an oxygen atom combines with 1.5 hydrogen atoms to form a water molecule. why is this inconsistent with dalton's atomic theory? an oxygen atom combines with 1.5 hydrogen atoms to form a water molecule. why is this inconsistent with dalton's atomic theory? atoms combine in 1:1 ratios to form compounds. atoms of one element cannot change into atoms of another element. oxygen and hydrogen atoms do not combine. atoms combine in simple, whole-number ratios to form compounds.
Answers
The statement is inconsistent with Dalton's atomic theory, which emphasizes that atoms combine in simple, whole-number ratios to form compounds.
The statement "an oxygen atom combines with 1.5 hydrogen atoms to form a water molecule" is inconsistent with Dalton's atomic theory because Dalton's atomic theory states that atoms combine in simple, whole-number ratios to form compounds.
According to Dalton's atomic theory, atoms are indivisible and retain their identity during chemical reactions. Atoms of different elements combine in fixed ratios to form compounds, and these ratios are expressed as simple whole numbers. For example, in the case of water (H2O), Dalton's theory would state that one oxygen atom combines with two hydrogen atoms in a 1:2 ratio.
The statement that an oxygen atom combines with 1.5 hydrogen atoms contradicts this fundamental principle of Dalton's atomic theory. Atoms cannot be divided into fractions or combine in non-whole-number ratios according to Dalton's theory.
Therefore, the statement is inconsistent with Dalton's atomic theory, which emphasizes that atoms combine in simple, whole-number ratios to form compounds.
Learn more about Dalton's atomic theory from the link given below.
https://brainly.com/question/1403872
#SPJ4
what is defference physical and chemical properties
Answers
Physical characteristics are those that can be seen or quantified without affecting a substance's chemical make-up. These characteristics include solubility, conductivity, solubility, density, melting and boiling defference
Substances are identified and described using bot defference their physical and chemical qualities, which are two separate categories of characteristics. Physical characteristics are those that can be seen or quantified without altering a substance's chemical make-up. Color, density, melting and boiling temperatures, solubility, and conductivity are a few examples of physical qualities. In contrast, a substance's behaviour during a chemical reaction or when it interacts with another material is described by its chemical characteristics.defferenceoxidation potential, and flammability are a few examples of chemical qualities. Physical and chemical attributes can be viewed or measured without affecting a substance's chemical makeup, but only when a chemical reaction takes place. This is the basic distinction between physical and
Learn more about defference here:
https://brainly.com/question/24078182
#SPJ4
a) Give the molar mass of Xenon
Answers
Answer:
131.293 u
Explanation:
difference between soap and detergent
Answers
Soap is potassium or sodium salts of a carboxylic acid attached to a long aliphatic chain. Detergent is the potassium or sodium salts of a long alkyl chain ending with a sulfonate group.
. One of the essential minerals in the human body is salt. How much salt (NaCl) is in the average adult human body?
Answers
Answer:
200g or 40 teaspoons
Explanation:
An average human, weighing about 50 pounds, has about 200 g or 40 tps of NACl
Compound A is heated with silver Powder and give compound B. Compound B is passed into the red hot copper tube at 600°C it gives Compound C of molecular formula C6H6.
i)identify Compound A and B with IUPAC name.
ii) How do you prove that the acidic nature of compound B?
iii) What happens when compound C reacts with bromine in the presence of catalyst FeCl3.
iv) Convert Compound C into Toulene.
Answers
Compound A is likely an organic halide, Compound B is a derivative of benzene, Compound C is benzene itself, and Compound C can be converted into toluene through a Friedel-Crafts alkylation reaction.
i) Compound A is an alkene.
When heated with silver powder, it undergoes oxidative cleavage to produce Compound B which is an aldehyde.
So the IUPAC names of Compound A and Compound B are ethene and ethanal, respectively.
ii) The acidic nature of Compound B can be proved by treating it with sodium hydrogen carbonate. If effervescence occurs, it is due to the evolution of carbon dioxide gas.
This indicates that Compound B is acidic in nature and reacts with a base to form salt and water.
iii) When Compound C (Benzene) reacts with bromine in the presence of catalyst FeCl3, Bromine water is decolorized to form a colorless solution.
This is an addition reaction that occurs due to the presence of an electron-rich benzene ring.
iv) Compound C (Benzene) can be converted into Toluene (Methylbenzene) through a process known as Friedel-Crafts Alkylation, where Benzene is allowed to react with Chloromethane (Methyl chloride) in the presence of Lewis acid catalyst, Aluminum chloride (AlCl3).
The resulting product is then heated to obtain Toluene (Methylbenzene).
The chemical reaction for the conversion of Benzene to Toluene is given below:C6H6 + CH3Cl → C6H5CH3 + HCl
For such more questions on Friedel-Crafts
https://brainly.com/question/30900581
#SPJ8