Ocean tides are caused by the___ attraction of the moon and sun.
Please help me. Asap
Answers
Answer:
Gravitational
Explanation:
please mark brainliest
Related Questions
will give free brainlist !! :)

Answers
Answer:
C is the correct answer
Explanation:
Answer:
C) 2920 J
Explanation:
have a happy day please can I have the brainlist I have bin trying to get one for a WEEK PLZ PLZ PLZ!!!
Why does the battery give a reading of 9V even though there are no electrons flowing around the circuit?
Answers
Answer:
because battery have it's own voltage in it's composition
The solute will dissolve quicker if the solute is more?
A. shaken.
B. saturated.
C. cooled.
D. settled.
Answers
Answer:
A) Shaken is the correct answer
Explanation:
The other three don't fit in with the solution if you think about it. When you shake something with lemonade crystals (Like a lemonade flavor packet in poured in a water bottle) it dissolves faster when you shake it rather than it sit on the bottom for 15 minutes! :) .
Hope this helps! :)
Answer:
The Person above me is correct
Explanation:
I got it right first try 100%
You are working a crime scene and discover a weapon. How do you pick up the weapon so you do not disturb
any latent fingerprints?
Answers
Answer:
you pick it up with gloves and make minimal contact with it
why do the words ‘shake well before using’ indicate that the fruit juice in a carton is a suspension?
Answers
Why do the words “Shake well before using” indicate that the fruit juice is a suspension?
Because some of the particles in a suspension are large enough that they will settle out over time.
Water can undergo a change of state when its energy changes at specific temperatures. Solid water, or ice, changes into liquid water when its energy increases at its melting point. a. Describe this process. b. Explain how the energy changes with each state.
Answers
Answer: The solid turns into liquid during melting.
Explanation:
a. The process of a solid turning into liquid is called melting. The solid ice turns into a liquid during the melting process. The solid molecules gain energy and flow as a liquid.
b. The energy of the molecules increases in the liquid state. When the ice turns into a liquid the energy of the molecules increases. The application of heat or excess temperature increases the energy of the molecules.
with the balanced equation : 4Fe + 3O2 = 2Fe2O3How many grams of Fe2O3 is produced when you start with 0.89 moles of iron?
Answers
Answer:
72.66g of Fe2O3 are produced.
Explanation:
1st) From the balanced equation we know that 2 moles of Fe2O3 are produced from 4 moles of iron (Fe). With a mathematical rule of three we can calculate the moles of Fe2O3 that will be produced from 0.89 moles of iron:
\(\begin{gathered} 4molesFe-2molesFe_2O_3 \\ 0.89molesFe-x=\frac{0.89molesFe*2molesFe_2O_3}{4molesFe} \\ x=0.445molesFe_2O_3 \end{gathered}\)Now we know that 0.455 moles of Fe2O3 are produced.
2nd) Now we have to convert 0.455 moles of Fe2O3 into grams, by using the molar mass of Fe2O3 (159.7g/mol):
\(0.455moles*\frac{159.7g}{1mole}=72.66g\)So, 72.66g of Fe2O3 are produced.
Please help solve this ASAP

Answers
Answer: i'd prolly say (e)
Explanation: if you ignore the letters and pay more attention to the symbols and numbers you can see that adding 18 to 2 makes 20 which is not an exponent and the 40 and 4 that are exponents put together makes 44 as dumb as it sounds it was a reasonable purpose behind it hope i did something
What happens in a
neutralisation reaction.
Answers
Answer:
A neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water. Salts are also formed in this reaction.
Explanation:
43a you are told that you have the option to add activated carbon (charcoal) to your recrystallization solution. why is it used?
Answers
Activated carbon, also known as charcoal, is commonly used in recrystallization because it is highly porous and has a large surface area that can effectively trap impurities.
This makes it an excellent adsorbent for organic contaminants, residual solvents, and other unwanted substances in the recrystallization solution. The addition of activated carbon to the solution prior to recrystallization helps to purify the desired compound and improve the overall yield of the reaction. Additionally, activated carbon is a highly effective filter that can remove any discoloration, odors, or other impurities that may be present in the solution. By improving the purity of the recrystallization solution, activated carbon can help to ensure that the final product is of a higher quality and has more consistent properties.
Learn more about charcoal:
brainly.com/question/3466681
#SPJ4
together, a pure gold ring and a pure titanium ring have a mass of 14.25 g. both rings are heated to 74.60 °c and dropped into 18.1 ml of water at 22.60 °c. the water and the rings reach thermal equilibrium at a temperature of 26.22 °c. the density of water is 0.998 g/ml. the specific heat capacity of water is 4.18 jg·°c, the specific heat capacity of gold is 0.129 jg·°c, and the specific heat capacity of titanium is 0.544 jg·°c. calculate the mass of each ring. chegg
Answers
By substituting Massgold +Masstitanium = 14.25 g, in the equation 276.6148 J = 0.129 J/g·°C * Massgold * 3.62 °C + 0.544 J/g·°C * Masstitanium * 3.62 °C, mass of each ring can be calculated by using the principle of heat transfer and apply the conservation of energy.
The heat lost by the rings is equal to the heat gained by the water.
Mass of gold ring + Mass of titanium ring = 14.25 g
Mass of water = 18.1 g
Initial temperature of water = 22.60 °C
Final temperature of water and rings = 26.22 °C
Density of water = 0.998 g/mL
Specific heat capacity of water (Cw) = 4.18 J/g·°C
Specific heat capacity of gold (Cg) = 0.129 J/g·°C
Specific heat capacity of titanium (Ct) = 0.544 J/g·°C
Step 1: Calculate the heat gained by the water:
Qwater = Cw * Masswater * ΔTw
Where ΔTw is the change in temperature of the water:
ΔTw = Final temperature - Initial temperature = 26.22 °C - 22.60 °C = 3.62 °C
Qwater = 4.18 J/g·°C * 18.1 g * 3.62 °C
Qwater = 276.6148 J
Step 2: Calculate the heat lost by the rings:
Qgold = Cg * Massgold * ΔTgold
Qtitanium = Ct * Masstitanium * ΔTtitanium
Since the gold and titanium rings are at the same temperature, their temperature change is the same:
ΔTgold = ΔTtitanium = ΔTw = 3.62 °C
Step 3: Set up the equation using the principle of heat transfer:
Qwater = Qgold + Qtitanium
276.6148 J = 0.129 J/g·°C * Massgold * 3.62 °C + 0.544 J/g·°C * Masstitanium * 3.62 °C
Step 4: Solve the equation to find the mass of each ring:
We have Massgold + Masstitanium = 14.25 g
Now we can solve the equation:
276.6148 J = 0.129 J/g·°C * Massgold * 3.62 °C + 0.544 J/g·°C * Masstitanium * 3.62 °C
Using the fact that Massgold + Masstitanium = 14.25 g, we can substitute and solve for the masses of the rings.
For more such questions on principle of heat transfer
https://brainly.com/question/9143372
#SPJ8
draw a segment of polybutadiene containing three monomer units. connect each end of the segment with a bond to one of the r groups. the r groups represent the connecting carbon atoms in the monomers adjacent to the segment. be sure to draw all hydrogen atoms.
Answers
This problem states polybutadiene as a polymer from which we have to draw three monomeric units, showing the rest of it with a general notation (R).
In this case, we need to consider that the monomeric unit in polybutadiene is 1,3-butadiene:
\(CH_2=CH-CH=CH_2\)
And takes the polymeric notation as:
\(-(CH_2=CH-CH=CH_2)_n-\)
Thus, since we need three monomeric units, we bind them as shown on the attached picture, which shows the 1st, 2nd and 3rd ones. Moreover, note the two R's, which stand for the rest of the polymer as a general notation.
Learn more:
https://brainly.com/question/2762383https://brainly.com/question/8156064
Help, please!! Been stuck on this question. Is it only neutral?

Answers
Answer:
neutral
Explanation:
.....................
when a candle burns both physical and chemical changes take place identify these changes . give another example of a familiar process in which both the chemical and physical changes take place
Answers
Answer:
Chemical Changes : The wax near flame burns and gives new substances like carbon dioxide, carbon soot, water vapour, heat and light. LPG is another example of a familiar process in which both the chemical and physical changes take place.
Answer:
When you sprinkle calcium chloride on snow, the snow will melt during the chemical change.
Explanation:
En 50 gr de de agua se disuelven 10 gr de NaCl con una densidad de 1,08 m/cm3. Hallar la M y la m a. 3,06 M y 3,06m b. 3,06 M y 3,4 m c 3,4 M y 3,06 m d 3,5 M y 3,4 m
Answers
Answer:
Opcion b. 3.06M y 3.4 m
Explanation:
Nuestra solución se compone de:
Soluto → NaCl (10 g)
Solvente → agua (50 g)
Primero, convertimos la masa de soluto a moles:
10 g . 1mol / 58.45g = 0.171 moles
Convertimos la masa de solvente, de g a kg → 50 g . 1kg/1000 g = 0.05 kg
m → molalidad (tipo de concentración que relaciona los moles de soluto en 1kg de solvente)
m = mol/kg → 0.171 mol/0.05kg = 3.42 m
Para calcular M, necesitamos el volumen de solución, y para eso aplicamos densidad. Antes, sumamos masa de soluto + masa de solvente, para obtener la masa de solución
50 g + 10 g = 60 g de solución
d = m /v → v = m /d → 60g / 1.08g/cm³ = 55.5 cm³
cm³ = mL
M = molaridad, la concentración que relaciona los moles de soluto en 1L de solución, pero que también podemos predecir como los milimoles de soluto en 1 mL de solución.
El volumen de solución está en mililitros → 55.5 mL
Convertimos moles a milimoles → 0.171 mol . 1000 mmol /mol = 171 mmol
M = 171 mmol /55.5 mL → 3.08 M
is soulubility maseruble physical property
Answers
A cube of steel has dimensions 0.2 mx 0.2 m * 0.2 m. What is
the volume of the cube in cubic centimeters?
cm
Answers
Answer: 8000
Explanation:
Which of the following is NOT a compound?
Water
Ammonia
Gold
Salt
Answers
The gold is not the compound.
The information related to the compound is as follows
It is the substance that contains the same type of molecules having 2 or more elements. It includes water, ammonia, salt, methane, carbon dioxide etc.In this, the elements should be bonded together.Therefore we can say that gold is not the compound.
Learn more related to the compound here: brainly.com/question/23334479
Define balanced forces(giving Brainly)
Answers
Answer:
Explanation:
Balanced forces are opposite in direction and equal in size.
Balanced forces are in a state of equilibrium.
Answer:
Balanced forces are forces whose net amount remains zero.
This can take place when two equal forces are acting in opposite direction.
Balanced forces don't make any change in the position of objects
What is crystallization how can you obtain bigger crystals
Answers
Answer:
Crystals that grow more slowly, tend to be larger. For crystals that were grown by slow cooling of the solvent: it usually improves the quality and size of the crystals, if the solution is slowly warmed up until alomst all crystals are dissolved again and than cooled dwon a second time very slowly.
Explanation:
rystallization or crystallisation is the process by which a solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some of the ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas.
what technological advancement was required for rutherford to conduct his experiment ?
Answers
Rutherford conducted a famous experiment called the gold foil experiment.
He took a skinny sheet of gold foil. He used a special gadget to shoot alpha particles undoubtedly charged debris on the gold foil. most debris passed straight thru the foil like the foil was now not there. They bombarded very skinny sheets of gold foil with fast-shifting alpha debris. Rutherford found that a small percent of alpha debris had been deflected at massive angles, which could be explained by using an atom with a very small, dense, definitely-charged nucleus at its middle.
Rutherford designed an experiment to apply the alpha particles emitted via a radioactive element as probes to the unseen global atomic structure. If Thomson changed into correct, the beam might move immediately through the gold foil. most of the beams went via the foil, however, some have been deflected. Ernest Rutherford postulated the nuclear structure of the atom, determined alpha and beta rays, and proposed legal guidelines for radioactive decay. In 1911, Rutherford, Marsden, and Geiger determined the dense atomic nucleus by bombarding a thin gold sheet with the alpha particles emitted through radium.
Learn more about Rutherford's experiment here:-https://brainly.com/question/14996029
#SPJ9
in a chemical reaction, glyceraldehyde-3-phosphate nad yields 1,3-bisphosphoglycerate nadh. in this reaction, what happened to nad ?
Answers
In the chemical reaction, NAD+ is reduced to NADH.
In the given chemical reaction, glyceraldehyde-3-phosphate is being oxidized, while NAD+ is being reduced. NAD+ (nicotinamide adenine dinucleotide) is a coenzyme that acts as an electron carrier in redox reactions. During the reaction, NAD+ accepts two electrons and a hydrogen ion (H+) to form NADH.
The oxidation of glyceraldehyde-3-phosphate results in the transfer of electrons to NAD+, leading to its reduction. As a result, NAD+ is converted to NADH, which now carries the extra electrons and a hydrogen ion.
You can learn more about chemical reaction at
https://brainly.com/question/11231920
#SPJ11
There are 8.421 g of dry ZnSO4 and
6.579 g H₂O in the sample.
Step 2: Determine the moles of water and anhydrous compound.
Zn = 65.38 g/mol, S = 32.07 g/mol,
H = 1.01 g/mol, O = 16.00 g/mol
How many moles of ZnSO4 are present?
[?] mol ZnSO4
Answers
The number of moles of H₂O is 0.36 mol.
The number of moles of LiClO₄ is 0.0521 mol.
What are moles?Mole is an important standard unit used for the measurement of large quantities of atoms, molecules, or other particles. One mole is equal to 6.022×10²³ units.
The number of moles of a substance is calculated by:
Moles =\(\frac{mass}{molar \;mass}\)
To find the number of moles of H₂O:
Mass of H₂O in the sample = 6.579 g
The molecular weight of H₂O = 18.02g
Moles = \(\frac{mass}{molar \;mass}\)
Moles = \(\frac{6.579 g}{18.02g}\)
Moles = 0.36
To find the number of moles of \(ZnSO_4\):
Mass of \(ZnSO_4\) in the sample = 8.421 g
The molecular weight of \(ZnSO_4\)= 161.47 g/mol
Moles =\(\frac{mass}{molar \;mass}\)
Moles = \(\frac{8.421 g}{161.47 g/mol}\)
Moles = 0.0521 moles
Learn more about moles here:
https://brainly.com/question/8455949
#SPJ1
A 10. 0-ml sample of 0. 75 m ch 3ch 2cooh is titrated with 0. 30 m naoh. what is the ph of the solution after 22. 0 ml of naoh have been added to the acid? [ k a(ch 3ch 2cooh) = 1. 3 × 10 –5]
Answers
The answer is-5.76
pH of a solution tells whether the solution is acidic or basic. It is calculated from the hydrogen ion concentrations in the solution as-
\(pH = -log [H^{+}]\)
How to determine [\(H^{+}\)] for a weak acid - strong base reaction?
In the given question, the acid is propanoic acid, \(CH_{3}CH_{2}COOH\) and the base is NaOH. When a weak acid and strong base reacts they form a buffer solution.The pH of the buffer solution is to be determined using Henderson-Hasselbalch equation which is expressed as:\(pH = pK_{a} + log\frac{[Conjugate\ base]}{[Acid]}\)The reaction between them is - \(CH_{3}CH_{2}COOH + NaOH\)→\(CH_{3}CH_{2}COONa + H_{2}O\)Calculate the initial moles of acid and base using their molarity and volume. \(Moles\ of\ CH_{3}CH_{2}COOH = Molarity\ of\ CH_{3}CH_{2}COOH\)×\(volume\ of\ CH_{3}CH_{2}COOH\ in\ L\) Moles of \(CH_{3}CH_{2}COOH\)= \(0.75 M\)×\(0.01\ L = 0.0075\ moles\)Similarly, Moles of \(NaOH\)= \(0.30 M\)×\(0.022 L = 0.0066 moles\) Moles of acid remaining = \(0.0075\ moles - 0.0066\ moles = 0.0009\ moles\) Total volume of solution = 10.0 mL + 22.0 mL = 32.0 mL\(pK_{a}= -log (K_{a}) = -log (1.3\)×\(10^{-5}) = 4.89\)Using Henderson-Hasselbalch equation, pH is calculated as-\(pH = 4.89 + log\frac{0.0066/32}{0.0009/32}\\\\pH = 4.89 + log (7.33)\\\\pH = 4.89 + 0.87\\\\ pH= 5.76\)
Hence, pH = 5.76
To learn more about pH of buffer solutions, visit:
https://brainly.com/question/9324316
#SPJ4
Which characteristics describe most nonmetals in the solid phase?
1
They are malleable and have metallic luster.
2
They are malleable and lack metallic luster.
3
They are brittle and have metallic luster.
4
They are brittle and lack metallic luster.
Answers
Answer:
4
Explanation:
its a non-metal, dont get confused and pick number 1- it may seem like that’s the answer but remember here we are talking abut the solid phase!
Periodic table is divided into three metals, non metals and metalloids. The non metals are kept on the right side of the periodic table. Th correct option is option D.
What are non metals?Non metals are the element that have property to gain electron. When any element gain electron then element attain negative charge and that element is called anion. The examples of non metals are Oxygen, nitrogen, carbon, Fluorine etc.
The properties of non metals are
Non metals are soft.
Non metals are not malleable that is they can be broken into thin sheets.
Non metals are not ductile they can not be broken into thin wires.
Non metals are brittle in nature that is they can be broken down easily.
Non metals are not lustrous.
Therefore the correct option is option D.
To learn more about non metals, here:
https://brainly.com/question/28650063
#SPJ2
Consider the reaction between barium chloride and sodium sulphate. If 25.0 mL of 0.75 M barium chloride react, how many grams of solid are produced?
Answers
n=0.75x0.025= 0.01875
m=n/M
m= 0.01875/(108+35.5x2) = 3.35625g
I’m pretty sure that’s the way to solve it but the answer is pretty small that kinda make me uncomfortable
The positively charged center in an atom is called as:A. NucleusB. NeutronsC. ProtonsD. Electrons
Answers
The positively charged center in an atom is called as Nucleus. It is made up of positively charged protons and neutral sub-atomic particle called as neutrons.
What is meant by protons ?
Every atom has a proton, a subatomic particle, in its nucleus.The particle possesses an electrical charge that is positive and opposite to the electron's.A subatomic particle with a negative charge is an electron. A subatomic particle having a positive charge is called a proton.The strong nuclear force holds protons together in the nucleus of an atom. A particular subatomic particle with no charge is the neutron.Although protons were once thought of as elementary particles, the Standard Model of particle physics now recognizes them as composite particles made up of three valence quarks, and they are grouped alongside neutrons as hadrons.To learn more about protons refer to
https://brainly.com/question/1805828
#SPJ4
Who was the chemist that devised a way to define acids and bases in 1887
Answers
Answer:
Svante Arrhenius
Explanation:
A gas mixture has three components: N2, F2, and He. Their partial pressures are 0.50 atm, 0.16 atm,
and 0.18 atm, respectively. What is the pressure of the gas mixture?
Enter your answer in the provided box.
atm
Answers
Answer:
What????????????????????????????
Explanation:
???
6. In which of the following will the bulb glow?
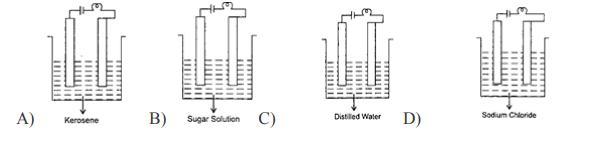
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later