No two electrons can have the same four quantum numbers is known as (the):_____.
a. heisenberg uncertainty principle.
b. pauli exclusion principle.
c. aufbau principle.
d. hund's rule.
Answers
The statement "No two electrons can have the same four quantum numbers" is known as the Pauli exclusion principle.
The Pauli exclusion principle, formulated by Wolfgang Pauli, is a fundamental principle in quantum mechanics that applies to particles with half-integer spin, such as electrons. According to this principle, no two electrons within an atom can have the exact same set of quantum numbers.
In quantum mechanics, each electron is described by a set of four quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). These quantum numbers provide information about the energy level, orbital shape, orientation, and spin state of an electron.
The Pauli exclusion principle states that for any given atom, no two electrons can have the exact same values for all four quantum numbers. This means that within a specific energy level, orbitals can accommodate a maximum of two electrons, and these two electrons must have opposite spins (one with ms = +1/2 and the other with ms = -1/2).
The Pauli exclusion principle plays a crucial role in determining the electronic configuration and behavior of atoms. It helps explain why electrons occupy different energy levels and orbitals, leading to the stability of atoms and the formation of chemical bonds.
In summary, the statement "No two electrons can have the same four quantum numbers" is known as the Pauli exclusion principle, which is a fundamental principle in quantum mechanics governing the behavior and arrangement of electrons in atoms.
learn more about Pauli exclusion here
https://brainly.com/question/30563805
#SPJ11
Related Questions
Arsenic has one stable isotope:75/33 As.
How many neutrons and protons are in a stable arsenic atom?
A. 33 neutrons and 42 protons B. 33 neutrons and 75 protons
C. 42 neutrons and 33 protons
D. 75 neutrons and 33 protons
Answers
what is the bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals?
Answers
The bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Bond order is defined as the number of electrons in bonding molecular orbitals minus the number of electrons in antibonding molecular orbitals divided by two. As a result, we may determine the bond order of this diatomic particle by the formula: Bond order = (number of bonding electrons - number of antibonding electrons) / 2
Bond order = (8 - 5) / 2
Bond order = 1.5.
This diatomic molecule, according to the bond order, is a stable molecule since the bond order is greater than 1, indicating that it is a double bond. The molecule has an overall bond strength that is greater than a single bond, but not as strong as a triple bond. So therefore he bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Learn more about bond order at:
https://brainly.com/question/30641030
#SPJ11
which of the states of matter have the most energy?
Answers
Plasma is the states of matter which have the most energy. Generally, there are four states of matter.
Plasma is one of four states of matter portrayed by the presence of a critical piece of charged particles in any mix of particles or electrons.[1] It is the most plentiful type of conventional matter known to man, being for the most part connected with stars, including the Sun Reaching out to the thin intracluster medium and potentially to intergalactic locales, plasma can be falsely created by warming an unbiased gas or exposing it to a solid electromagnetic field.
The presence of charged particles makes plasma electrically conductive, with the elements of individual particles and plainly visible plasma movement administered by aggregate electromagnetic fields and exceptionally delicate to remotely applied fields.The reaction of plasma to electromagnetic fields is utilized in numerous cutting edge gadgets and advances, for example, plasma TVs or plasma etchin
To know more about plasma,visit here:
https://brainly.com/question/18207038
#SPJ4
Dalton's experiments advanced our understanding of the atom without:____.
Answers
Dalton's experiments advanced our understanding of the atom without the use of sophisticated scientific tools that are available today.
In the late 18th and early 19th centuries, Dalton carried out a series of experiments that led him to propose the atomic theory, which revolutionized the way scientists thought about matter.
Despite his lack of access to modern instruments, Dalton was able to make several important observations about the behavior of gases, which led him to propose that all matter is composed of small, indivisible particles called atoms. Dalton also suggested that atoms of different elements have different properties and combine in fixed ratios to form compounds.
While Dalton's atomic theory has been modified and refined over the years, his experiments laid the foundation for our understanding of the structure and behavior of matter. Dalton's work demonstrates the importance of careful observation and deduction in scientific inquiry, even in the absence of advanced technology.
Learn more about atom:
brainly.com/question/1566330
#SPJ4
Determine the number of grams in 0.5 moles of H2 S04
Answers
Mention two substance that sublines
Answers
Familiar substances that sublime readily include iodine , dry ice , menthol, and camphor. Sublimation is occasionally used in the laboratory as a method for purification of solids, for example, with caffeine.
the proton nmr spectrum of an aromatic compound, c8h8br2, includes two methyl singlets. its proton-decoupled 13c nmr spectrum displays a total of six peaks. of the following compounds, which structure best fits these data?
Answers
The structure that best fits the given data is 1,4-dibromobenzene.
The presence of two methyl singlets in the proton NMR spectrum indicates the presence of two methyl groups in the compound. This suggests the presence of a substituent attached to the benzene ring.
The proton-decoupled 13C NMR spectrum displays six peaks, indicating the presence of six distinct carbon environments. In 1,4-dibromobenzene, there are two carbon atoms attached to the methyl groups, which gives two peaks. The benzene ring itself has four unique carbon environments, each with a different chemical shift, resulting in four additional peaks.
The structure of 1,4-dibromobenzene matches the data because it contains two methyl groups and displays a total of six peaks in the proton-decoupled 13C NMR spectrum, consistent with the given information.
To learn more about NMR spectrum, here
https://brainly.com/question/30465398
#SPJ4

An ideal ga (which i i a hypothetical ga that conform to the law governing ga behavior) confined to a container with a male piton at the top. (Figure 2) A male wire i attached to the piton. When an external preure of 2. 00 atm i applied to the wire, the ga compree from 6. 40 to 3. 20 L. When the external preure i increaed to 2. 50 atm , the ga further compree from 3. 20 to 2. 56 L. In a eparate experiment with the ame initial condition, a preure of 2. 50 atm wa applied to the ideal ga, decreaing it volume from 6. 40 to 2. 56 L in one tep. If the final temperature wa the ame for both procee, what i the difference between q for the two-tep proce and q for the one-tep proce in joule?
Answers
An ideal gas is a gas composed of a large number of identical molecules that obey the ideal gas law.
What do you mean by Gas?
Gas is a state of matter made of atoms and molecules that are not bound together, allowing them to move freely and take the shape and volume of their container. It is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
The heat exchanged in a two-step process is given by
q = CpΔT + (P1V1 – P2V2)
where Cp is the specific heat capacity of the gas, ΔT is the change in temperature, P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
For the two-step process,
q = CpΔT + (2.00 atm x 6.40 L – 2.50 atm x 3.20 L)
For the one-step process,
q = CpΔT + (2.50 atm x 6.40 L – 2.50 atm x 2.56 L)
The difference in q for the two-step process and the one-step process is
q2 – q1 = (2.50 atm x 3.20 L – 2.00 atm x 6.40 L)
q2 – q1 = (7.68 L atm – 12.80 L atm)
q2 – q1 = -5.12 L atm
Since 1 L atm = 101.3 J, the difference in q for the two-step process and the one-step process is
q2 – q1 = -5.12 L atm x 101.3 J/L atm
q2 – q1 = -517.4 J.
Hence, the difference is -517.4 J.
To know more about Gas,
https://brainly.com/question/27870704
#SPJ4
WHICH IS A PROPERTY OF ALL BASES?
A. Their formulas include H3O+ ions
B. They increase the OH- concentration in solution
C. They increase the H3O+ concentration in solution
D. Their formulas include OH- ions
Answers
According to the research, the correct option is B. They increase the OH- concentration in solution is a property of all bases.
What is a base?It is the substance that has alkalinity that, when in solution, increases the concentration of hydroxyl ions (OH-) and establishes combinations with acids for the development of salts.
They are soluble in water and usually have a bitter taste and by providing hydroxyl ions to the medium, they are capable of neutralizing acids.
Therefore, we can conclude that according to the research, the correct option is B. They increase the OH- concentration in solution is a property of all bases.
Learn more about bases here: https://brainly.com/question/16226890
#SPJ1
Which of these is the most common greenhouse gas released by
outgasing?
a) Nitrogen
b) Molecular Oxygen (O2)
c) Carbon Dioxide (CO2)
d) Water Vapor
e) Ozone (O3)
Answers
which of the following molecules or ions contain an oxygen atom with a positive formal charge?
a. CO2
b. CO
c. CO2^-2
d. H2O
Answers
Carbonate ions \(CO_2^{-2}\) contain an oxygen atom with a favorable elevated charge. Thus, option C is correct.
The carbonate ion is a compound that is formed by sharing valency shell electrons of Carbon and Oxygen elements. After forming, the oxygen atom in the middle has a positive formal charge. This is because each oxygen atom in the carbon will be assigned a formal charge of -1.
In this reaction, when the compound is double-bonded, the oxygen atom in the middle has only six electrons in the valence shell instead of eight electrons, resulting in a positive formal charge of +2 after the reaction.
To learn more about carbonate ion
https://brainly.com/question/31215748
#SPJ4
PLZZZ HELP BRAINILEST (science)

Answers
Answer:
i cannot see the question due to the flash you used
Explanation:
1. Multiple Choice
How does altitude affect climate?
A.
The higher the altitude, the colder the climate will be.
B.
The lower the altitude, the colder the climate will be.
C.
The higher the altitude, the hotter the climate will be.****
D. Altitude is not related to climate.
2. How does latitude affect climate?
A.
Areas near the equator have cooler climates than areas far from the equator.
B.
Areas near the equator have warmer climates than areas far from the equator.
C.
Areas near the equator have four seasons.****
D.
Areas near the equator do not have any type of precipitation.
3. How does wind affect precipitation?
A.
Wind does not have any effect on precipitation.
B.
Winds that blow from over the land contain a lot of water vapor that will cause precipitation.****
C.
Winds that blow inland from oceans or large lakes contain very little water vapor that will cause precipitation.
D. Winds that blow inland from oceans or large lakes contain a lot of water vapor that will cause precipitation.
4. Sea and land breezes over a large region that change direction with the seasons are called
A.
windward.
B.
leeward.
C.
monsoons.****
D. storms.
(****= my answer)
plz cheak my answers!?
Answers
Answer:
#1 A
#2 B
#3 C
#4 c
Explanation:
#1 can give reference to a mountain the higher the altitude the colder it will get
#2 talking about climate not seasons per say. focus on climate answers
#3 wind carry moisture from the sea on to land which cause precipitation
#4 Monsoons is correct
If you need to produce 66 grams of carbon dioxide, how many liters of water vapor would you produce as a by product?

Answers
The question requires us to calculate the amount of vapor water produced as a by-product when 66g of carbon dioxide are obtained from the combustion of propane.
Considering the combustion of propane (C3H8), we have the following reaction:
\(2C_3H_8+9O_2\to4CO_2+2CO_{}+8H_2O_{(v)}\)IFrom the reaction, we can see that the stoichiometric relationship between C3H8 and water (H2O) is as follows:
2 mol C3H8 ---------- 8 mol H2O
Then, to calculate the amount of water produced as a by-product, we'll need to determine the amount of reactant needed to produce 66g of CO2.
Since the molar mass of CO2 is 44g/mol and considering the reaction written above, we can write:
2 mol C3H8 ---------- 4 mol CO2
x ---------- (66g/44g) = 1.5 mol CO2
Solving for x, we have that 0.75 mol of C3H8 are required to produce 66g of CO2.
Now, we calculate the amount of water that should be obtained from 0.75 mol of C3H8:
2 mol C3H8 ---------- 8 mol H2O
0.75 mol C3H8 ----- y
Solving for y, we have that 3 moles of water will be obtained as a by-product.
At last, we convert the calculated amount of vapor water into its volume considering the Standard Temperature and Pressure conditions (STP), where 1 mol of a gas corresponds to 22.4 L of the same gas:
1 mol vapor H2O ---------- 22.4 L vapor H2O
3 mol vapor H2O --------- z
Solving for z, we have that 67.2 L of vapor water will be obtained as a by-product when 66g of CO2 are produced from the combustion of propane.
In the previous step, you determined
0.25 mol HCI reacts. The molar mass
of Mg is 24.31 g/mol.
What mass of Mg is required?
PLEASE HELP ASAP

Answers
Approximately 3.04 grams of magnesium would be required to react with 0.25 moles of hydrochloric acid.
To determine the mass of Mg required, we need to use the balanced chemical equation for the reaction between hydrochloric acid (HCl) and magnesium (Mg):
2HCl + Mg → MgCl2 + H2
From the balanced equation, we can see that 2 moles of HCl react with 1 mole of Mg. Therefore, if 0.25 mol of HCl reacts, we would need half of that amount, which is 0.125 mol of Mg.
To calculate the mass of Mg required, we need to multiply the number of moles of Mg by its molar mass. The molar mass of Mg is given as 24.31 g/mol. Therefore, the mass of Mg required can be calculated as follows:
Mass of Mg = Number of moles of Mg × Molar mass of Mg
Mass of Mg = 0.125 mol × 24.31 g/mol
Mass of Mg = 3.04 g
For such more questions on moles
https://brainly.com/question/19964502
#SPJ8
In the product 6o2, what does the coefficient mean?.
Answers
Answer:
It means there are 6 O2 molecules
Explanation:
Which of the following outcomes is a benefit of conventional agriculture?
Answers
Answer:
Preserving soil, water and energy resources has enabled modern farming to grow food sustainably on the same land for more than 100 years. ... Modern, conventional agriculture has key sustainability benefits in terms of land use, reduced soil erosion and water protection.
Explanation:
hope it helps you
what are the subatomic particles by which atom made of?
Answers
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electrons.
Explanation:
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electron
8. Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a ___________________ solution.
Answers
Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a hypertonic solution.
What is a hypertonic solution?A hypotonic solution is described as a solution that has lower osmotic pressure than another solution to which it is compared.
The concept of tonicity helps us understand that the saltwater we use to brine the turkey is typically considered hypertonic solution because it has a greater concentration of solutes than the liquid found inside the cells.
Learn more about hypertonic solutions at: https://brainly.com/question/4237735
#SPJ1
Which is an example of chemical weathering?
Answers
Connect It Imagine that you can see the particles of ice, liquid water, and water vapor. Describe how these three states of water differ.
Answers
ice particles have a strong bonding and arranged regularly so the atom is very close bond to each other in regular arrangement.
the liquid bonding is weaker than ice and the movement of atom is slightly flow around so the atom is slighty close to each other,
vapor bonding is weaker than the liquid and the atom movement is in all direction so the atom of water in this state is far apart from each other and move in all direction
Gas is confined in a metal tank represented by the figure below. At 283.2K 283.2 K , the gas exerts a pressure of 7.571atm 7.571 a t m . After heating the tank, the pressure of the gas increases to 12.846atm 12.846 atm.
Answers
The gas in the tank has a final temperature of roughly 480.5 K.
Why is the gas under pressure?Gas molecules interacting with an object's surface generate force, which is what creates gas pressure. Although though there is very little force involved in each impact, any surface with a sizeable area is subject to many of them quickly, which can lead to a high pressure.
As per the ideal gas law,
PV = nRT
We can write: Provided that the volume and quantity of moles of gas remain constant.
P1/T1 = P2/T2
where the initial pressure and temperature are P1 and T1, and the ultimate pressure and temperature are P2 and T2.
With the values provided, we have:
P1 = 7.571 atm
T1 = 283.2 K
P2 = 12.846 atm
We can solve for T2:
T2 = (P2/P1) * T1
= (12.846 atm / 7.571 atm) * 283.2 K
= 480.5 K
To know more about gas pressure visit:-
https://brainly.com/question/9099789
#SPJ1
Zinc chemically bonds with sulfur in the presence of heat to form zinc sulfide
Answers
The formation of Zinc sulfide from 'Zn' and 'S' is a redox reaction, because it involves both oxidation and reduction reactions. So zinc bonds with sulfur to form zinc sulfide.
What is Zinc sulfide?The zinc sulfide also called the zincblende is a white or yellowish crystal and it is insoluble in water as well as denser than water. It is manufactured by the combustion of a mixture of zinc and sulfur.
The compound 'ZnS' decomposes in the presence of oxidizing agents and acids. The reaction is given as:
Zn + S → ZnS
The oxidation reaction is:
Zn - 2e⁻ → Zn²⁺
The reduction reaction is:
S + 2e⁻ → S⁻²
To know more about zinc sulfide, visit;
https://brainly.com/question/28607192
#SPJ1
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt(III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane−1,2−diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane−1,2−diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
Answers
The formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt(III) chloride: [Co(NH₃)₄(H₂O)₂]Cl₃;
(ii) Potassium tetracyanonickelate(II): K₂[Ni(CN)₄];
(iii) Tris(ethane−1,2−diamine) chromium(III) chloride: [Cr(en)₃]Cl₃;
(iv) Amminebromidochloridonitrito-N-platinate(II): [Pt(NH₃)₂BrCl(NO₂)];
(v) Dichloridobis(ethane−1,2−diamine)platinum(IV) nitrate: [PtCl₂(en)₂]NO₃;
(vi) Iron(III) hexacyanoferrate(II): [Fe(H₂O)₆][Fe(CN)₆].
(i) Tetraamminediaquacobalt(III) chloride: [Co(NH₃)₄(H₂O)₂]Cl₃
The coordination sphere of the complex contains cobalt (III) ion surrounded by four ammine (NH₃) ligands and two aqua (H₂O) ligands. The counter ion, chloride (Cl⁻), is outside the coordination sphere and hence written in square brackets.
(ii) Potassium tetracyanonickelate(II): K₂[Ni(CN)₄]
The coordination sphere of the complex contains nickel (II) ion surrounded by four cyano (CN⁻) ligands. The two potassium (K⁺) ions are outside the coordination sphere and hence written separately.
(iii) Tris(ethane−1,2−diamine) chromium(III) chloride: [Cr(en)₃]Cl₃
The coordination sphere of the complex contains chromium (III) ion surrounded by three ethane-1,2-diamine (en) ligands. The counter ion, chloride (Cl⁻), is outside the coordination sphere and hence written in square brackets.
(iv) Amminebromidochloridonitrito-N-platinate(II): [Pt(NH₃)₂BrCl(NO₂)]
The coordination sphere of the complex contains platinum (II) ion surrounded by two ammine (NH₃) ligands, one bromido (Br⁻) ligand, one chlorido (Cl⁻) ligand, and one nitrito (NO₂⁻) ligand.
(v) Dichloridobis(ethane−1,2−diamine)platinum(IV) nitrate: [PtCl₂(en)₂]NO₃
The coordination sphere of the complex contains platinum (IV) ion surrounded by two ethane-1,2-diamine (en) ligands and two chlorido (Cl⁻) ligands. The counter ion, nitrate (NO₃⁻), is outside the coordination sphere and hence written in square brackets.
(vi) Iron(III) hexacyanoferrate(II): [Fe(H₂O)₆][Fe(CN)₆]
The coordination sphere of the complex contains two entities. The first entity contains iron (III) ion surrounded by six aqua (H₂O) ligands. The second entity contains hexacyanoferrate (II) ion, which is coordinated to the first entity through cyanide (CN⁻) ligands. The two entities are separated by a square bracket.
To know more about coordination compounds, refer to the link below:
https://brainly.com/question/29766591#
#SPJ11
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
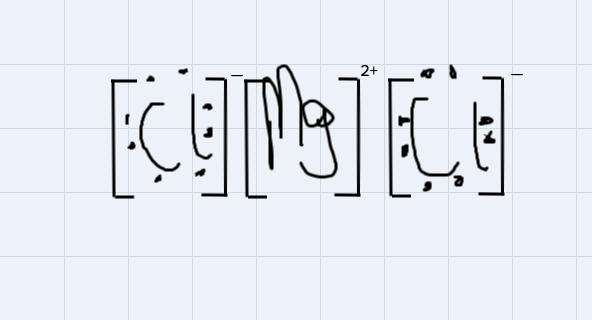
When naming an acid, which of the following is true?
chlorite changes to chloric acid
chlorate changes to chloric acid
none of these is true
chlorate changes to chlorous acid
Answers
Answer:
chlorate changes to chlorous acid
a bond in which only one pair of
electrons is shared?
Answers
Answer:
A single covalent bond
Explanation:
A single covalent bond is when only one pair of electrons is shared between atoms. A sigma bond is the strongest type of covalent bond, in which the atomic orbitals directly overlap between the nuclei of two atoms.
Answer:
Single covalent bond.
Explanation:
Single covalent bond is the type of bond in which only one pair of electrons is shared between atoms.
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807Can someone please please help me?? I will give you BRAINIEST

Answers
Answer:
a) 9.0 B. 14
Explanation:
6*4-15=9 we need 2 sig figs so 9.0
18/2.0+5.3=14.3 we need 2 sig figs so 14
but not 100%
In Part 1, draw the product that results from the mechanism arrows provided. In Part 2, indicate whether the elementary step is reversible.
Answers
In Part 1 of the question, we are asked to draw the product that results from the provided mechanism arrows. This involves analyzing the reaction steps and understanding the changes that occur during the reaction.
In Part 2, we need to determine whether the elementary step is reversible, meaning if the reaction can proceed in both the forward and reverse directions.
In Part 1, drawing the product resulting from the provided mechanism arrows requires a careful analysis of the reaction steps. The mechanism arrows indicate the movement of electrons and the formation or breaking of bonds. By following the arrows and considering the reactivity of the reactants, we can determine the resulting product(s) of the reaction. It is important to pay attention to the regioselectivity and stereochemistry of the reaction, if applicable.
In Part 2, determining whether the elementary step is reversible involves considering the nature of the reaction and the thermodynamics of the process. Reversible elementary steps involve both forward and reverse reactions occurring under certain conditions. Factors such as energy barriers, equilibrium constants, and reaction conditions influence reversibility. By analyzing these factors, we can determine whether the elementary step is reversible or if the reaction predominantly proceeds in one direction.
Overall, these two parts require a detailed understanding of the reaction mechanism, electron movement, and the factors affecting reversibility to accurately draw the product and determine the reversibility of the elementary step.
Learn more about movement here;
brainly.com/question/11223271
#SPJ11
In Part 1, draw the product that results from the mechanism arrows provided. In Part 2, indicate whether the elementary step is reversible. LCH3 4th attempt
Consider the equations below.
H₂ → 2H
CHA+HC2H5
C2H5 + HC2H6
When these equations are added together, what will the overall equation be?
C₂H4+H₂C₂H
H2 + CH4 + 2H + C2H5+ → 2H + CH3 + CHE
C2H4 +C2H4 + H2>C2H5 + C2H5
Answers
Answer:
a
Explanation:
The overall equation is \(C_2H_4 + H_2 ---- > C_2H_6\).
What is Chemical Equation?A chemical equation is defined as a symbolic representation of a chemical reaction in the form of symbols and formulas, in which reactant elements are given on the left and product units on the right.
For example,
\(NaOH+ HCl ---- > NaCl+ H_2O\)
In this, reactants are converted to products which is symbolized by a chemical equation. For example, iron (Fe) and sulfur (S) combine to form iron sulfide (FeS).
Fe(s) + S(s) → FeS(s)
here, the plus sign indicates that iron reacts with sulfur.
Thus, the overall equation is \(C_2H_4 + H_2 ---- > C_2H_6\).
Learn more about Chemical Equation, here:
https://brainly.com/question/30087623?
#SPJ2