Na2S+2AgNO3 = Ag2S + NaNO3 If 2.86g of Ag2S are actually produced by a reaction between an excess of Na2S and 4.27g of AgNO3 then what is the percent yield of Ag2S
A. 3.15%
B. 45.9%
C. 61.2%
D. 91.0%
Answers
Answer:
D. 91.0%
Explanation:
Hello,
In this case, for the given chemical reaction:
\(Na_2S+2AgNO_3 \rightarrow Ag_2S + 2NaNO_3\)
Next, since silver nitrate (molar mass 169.87 g/mol) is in a 2:1 molar ratio with silver sulfide (molar mass 247.8 g/mol), we compute its theoretical yield as shown below:
\(m_{Ag_2S}^{theoretical}=4.27gAgNO_3*\frac{1molAgNO_3}{169.87gAgNO_3} *\frac{1molAg_2S}{2molAgNO_3}*\frac{247.8gAg_2S}{1molAg_2S}\\\\m_{Ag_2S}^{theoretical}=3.11gAg_2S\)
Next, we compute the percent yield as:
\(Y=\frac{m_{Ag_2S}^{actual}}{m_{Ag_2S}^{theoretical}}*100\% =\frac{2.86g}{3.11g} *100\%\\\\Y=91.0%\)
Hence, answer is D. 91.0%.
Best regards.
Related Questions
What is the correct order of steps to turn water into electrical energy?
Answers
1. Water flows through the penstock
2. The water then spins the blades in a turbine which, in turn, spins a generator that ultimately produces electrical energy
How do we convert water into electrical energy?Hydropower, or hydroelectric power, is one of the oldest and largest sources of renewable energy, which uses the natural flow of moving water to generate electrical energy. The moving water is passed through a pipe also known as penstock, it then turns the blade of the turbine. which then rolls or spin a generator thereby electricity is produced.
The work of the turbine is to convert the kinetic energy of the running water into useable energy.
learn more about conversion of water to electrical energy from
https://brainly.com/question/18776929
#SPJ1
what is thr approximate molar concetrations of na ions uworld
Answers
The approximate molar concentration of Na+ ions can be determined by considering the concentration of a sodium-containing compound or the concentration of Na+ in a solution is 1M
The molar concentration of Na+ ions can vary depending on the context. If you have a specific sodium-containing compound, you can determine the molar concentration of Na+ ions by considering its formula and the stoichiometry of the compound. For example, if you have a 1 M solution of sodium chloride (NaCl), the molar concentration of Na+ ions would be 1 M.
In a more general sense, if you have a solution containing sodium ions (Na+), you can determine the approximate molar concentration of Na+ ions by measuring the concentration of a sodium-containing compound or using analytical techniques such as ion-selective electrodes or spectrophotometry.
It's important to note that the molar concentration of Na+ ions can vary depending on the specific solution or compound being considered. Therefore, it is necessary to specify the particular context or compound to obtain a more accurate determination of the molar concentration of Na+ ions.
Learn more about concentration here
https://brainly.com/question/32241761
#SPJ11
The Complete question is
what is the approximate molar concetrations of Na ions in NaCl?
convert 7.9 x 10^22 atoms of Ca to grams
Answers
Answer:
Mass = 5.24 g
Explanation:
Given data:
Number of atoms of Ca = 7.9×10²² atoms
Mass of Ca = ?
Solution:
First of all we will calculate the number of moles by using Avogadro number.
1 mole contain 6.022×10²³ atoms
7.9×10²² atoms × 1 mol /6.022×10²³ atoms
1.31 ×10⁻¹ mol
0.131 mol
Mass of Ca:
Mass = number of moles × molar mass
Mass = 0.131 mol × 40 g/mol
Mass = 5.24 g
Determine the number of seconds in one week.
Answers
Answer:
604,800 seconds in a week
Explanation:
86,400 seconds in a day
86,400 × 7 = 604,800
Help me with this question please

Answers
Answer:
a. Oxidising agent: Cl₂
b. Reducing agent: NaBr
c. Oxidised: NaBr
d. Reduced: Cl₂
e. Oxidation numbers before reaction: Cl= 0, Na= +1, Br= -1
f. Oxidation numbers after reaction: Cl= -1, Na= +1, Br= 0
Explanation:
Oxidising agents reduces themselves, oxidising other elements/compounds.
Reducing agents oxidise themselves, reducing other elements/compounds.
Oxidation is the loss of electrons or an increase in oxidation number.
Reduction is the gain of electrons or decrease in oxidation number.
In an experiment, sulfuric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each flask became gradually invisible due to the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown.
Experimental Record
Flask Volume of H2SO4 Volume of Sodium Thiosulfate Volume of Water Time
1 5 mL 50 mL 0 mL 19 seconds
2 5 mL 40 mL 10 mL
3 5 mL 30 mL 20 mL
4 5 mL 20 mL 30 mL
Based on factors that affect the rates of chemical reactions, which of the following would describe the trend expected in the table?
Answers
Based on factors that affect the rates of chemical reactions, the trend expected in the table is that as the volume of sodium thiosulfate in water increases, 1) 5 mL 50 mL 0 mL 19 seconds
what is sodium thiosulfate ?
Sodium thiosulfate (Na2S2O3) is a salt composed of sodium, sulfur, and oxygen atoms. It is a colorless, crystalline solid that dissolves in water and has a variety of uses in different fields.
One of the most common uses of sodium thiosulfate is as a photographic fixer, where it is used to remove unexposed silver halide from photographic films and papers. It is also used as a neutralizing agent for chlorine and other oxidizing agents in water treatment, as a dechlorinating agent in the treatment of wastewater, and as a reagent in the laboratory for different chemical reactions.
Based on factors that affect the rates of chemical reactions, the trend expected in the table is that as the volume of sodium thiosulfate in water increases (
As the concentration of sodium thiosulfate increases, the frequency of effective collisions between the reactant molecules and the likelihood of successful collisions increases, resulting in a faster reaction rate. Therefore,
Flask 1 -- 1) 5 mL 50 mL 0 mL 19 seconds(with the highest concentration of sodium thiosulfate) should have the shortest time taken for the cross to become invisible, and
Flask 4 -- 4) 5 mL 20 mL 30 mL(with the lowest concentration of sodium thiosulfate) should have the longest time taken for the cross to become invisible.
To learn more about sodium thiosulfate follow the given link: https://brainly.com/question/14960336
#SPJ1
Convert 3 J into kj. Please show work and explain.
Answers
The kilojoule is 0.0003
The joules is the smallest quantity than the kilojoule.when we convert the small quantity into the large quantity than we used the decimals.So 3J is the small quantity and the kilojoule contains the 1000 joule.1 kilojoule contains 1000 joules.if we convert this values from joule to kilojoule, the value is like this1 joule contains 0.0001 kilojoulessimilarly,3 joule contain0.0003 kilojoules.So the joule is the unit of energy (heat energy, thermal energy etc.)It is defined as the Amount of energy is transferred to the object when the amount of force is used to displace the motion.the joule unit is expressed in J = kg×m²/s = NmWhen you convert the small quantity to large quantity then we always use the decimals.
Learn more about conversion quantity here
https://brainly.com/question/18559676
#SPJ9
A substance that keeps its shape because its particles can't flow freely is a(n) _____________.
Answers
The substance that keeps its shape because its particles cannot flow freely is known as a solid. Solids have a fixed shape and volume because the particles are tightly packed together and cannot move freely.
The particles in solids are arranged in a specific pattern that gives them a definite shape. This pattern of arrangement is referred to as the crystal lattice structure.Solids are distinguished from liquids and gases by their ability to maintain their shape and volume. Liquids, on the other hand, take the shape of their container because their particles can flow freely, but they still have a fixed volume. Gases, on the other hand, can flow freely and can also expand or contract to fill the entire space available to them.In summary, a substance that keeps its shape because its particles cannot flow freely is a solid. This characteristic is due to the tight packing of particles and the arrangement of the crystal lattice structure. Solids are one of the three states of matter and are distinguished from liquids and gases by their fixed shape and volume.
Learn more about particles here
https://brainly.com/question/27911483
#SPJ11
What is the relation for entropy change for reversible process?
Answers
If the process is irreversible, the entropy change may be positive, negative, or zero, depending on the direction of heat flow.
The relation for entropy change for a reversible process is given by the equation ΔS = Qrev/T, where ΔS is the change in entropy, Qrev is the heat absorbed or released during the reversible process, and T is the temperature at which the process occurs. In a reversible process, the entropy change is positive for an increase in temperature and negative for a decrease in temperature. This equation is important in thermodynamics because it allows us to calculate the change in entropy for a reversible process and determine the maximum efficiency of a heat engine.
To learn more about entropy click here https://brainly.com/question/13999732
#SPJ11
A group of scientists have obtained some experimental results.
How would the group best find out whether their study is worth more time and resources?
Answers
To determine whether their study is worth more time and resources, the group of scientists can consider the following steps: Evaluate the significance of the results, Peer review and expert feedback, Conduct additional experiments or studies.
Evaluate the significance of the results: The scientists should carefully analyze the experimental results to assess their scientific and practical importance.
They need to determine if the outcomes align with the research goals, contribute to existing knowledge, or have potential applications in their field. If the results demonstrate promising findings or address important research questions, it indicates that further investigation could be worthwhile.
Peer review and expert feedback: Submitting the study for peer review is crucial for obtaining feedback from other experts in the field. Peer reviewers can provide valuable insights, identify potential limitations, and offer suggestions for improvement. Constructive feedback from peers is essential in assessing the scientific rigor and potential impact of the study.
Conduct additional experiments or studies: If the initial results show promise but require further validation or replication, the scientists may need to design additional experiments or studies to strengthen their findings.
These follow-up experiments can provide more robust evidence, address any potential limitations, or explore related aspects of the research question.
For more such questions on scientists visit:
https://brainly.com/question/29886197
#SPJ8
5) When rain falls to the earth, it just doesn't sit there. Instead, the rain water starts moving. Some of the precipitation seeps into the ground to replenishing groundwater. However, most of
it flows downhill as runoff. Runoff is important in filling rivers and lakes, but it also changes the landscape by erosion and can be a powerful mover. Flowing water is a powerful force,
capable of moving boulders and carving out canyons. Runoff has an impact on streams and rivers, but it also has an impact on the ocean system. What can you infer about the impact of
runoff on Ocean life? Choose ALL that apply.
A) Runoff drops a lot of sediment in the ocean, decreasing salinity.
B) Runoff builds up the coastline, depositing sediment, disrupting habitats of marine wildlife.
Runoff can lead to overgrowth of algae that can cause oxygen deprivation and create a dead zone.
D) Runoff carries pollutants into the ocean, causing illness and death of vulnerable marine populations.
) Runoff can carry bacteria, viruses and other pathogens from pet and livestock waste that can harm shellfish and other marine organisms.
Answers
Answer: Rain evaporates and this same process happens over and over again.
Explanation:
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
Assume that 8.5 L of iodine gas (I2) are produced at STP according to the following balanced equation:
2KI (aq) + Cl2 (g) --> 2KCl (aq) + I2 (g)
a ) How many moles of KI were used?
b ) How many grams of KI were used
any help is appreciated:(((
Answers
molar mass I equal to the lenses of gravity
Answer:
a) 0.799 mol (3 s.f.)
b) 133 g (3 s.f.)
Explanation:
2KI (aq) + Cl₂ (g) → 2KCl (aq) + I₂ (g)
1) Check if the equation has been balanced.
• The equation is already balanced in this question as the number of atoms of each element is equal on both sides.
Part (a)
2) Convert 8.5 L of I₂ into number of moles.
At STP (standard temperature and pressure), 1 mole of gas occupies 22.4 L.
Amount of I₂ gas produced
= 8.5 ÷22.4
= 0.37946 mol (5 s.f.)
3) Identify the relationship between I₂ (g) produces and KI (aq) used in terms of mole ratio.
From the balanced equation, the mole ratio of KI (aq) used to the amount of I₂ (g) produced is given as:
KI (aq): I₂ (g)
= 2 :1
This is obtained by comparing the coefficients of KI (aq) and I₂ (g) after the equation is balanced.
The mole ratio tells us that for every 1 mole of I₂ (g) produced, 2 moles of KI (aq) is needed.
4) Calculate the amount of KI (aq) used.
1 mole of I₂ (g) ----- 2 moles of KI (aq)
0.37946 mol of I₂ (g) ----- 2(0.37946)= 0.79852 mol of KI (aq) (5 s.f.)
Thus, 0.799 mol of KI were used. (3 s.f.)
Part (b)
5) Convert number of moles into weight.
Mole ×Mr= Weight in grams
Mr stands for relative formula mass and can be calculated with the use of a periodic table.
Weight of KI (aq) used
= 0.79852 ×(39.1 +127)
= 0.79852 ×166.1
= 133 g (3 s.f.)

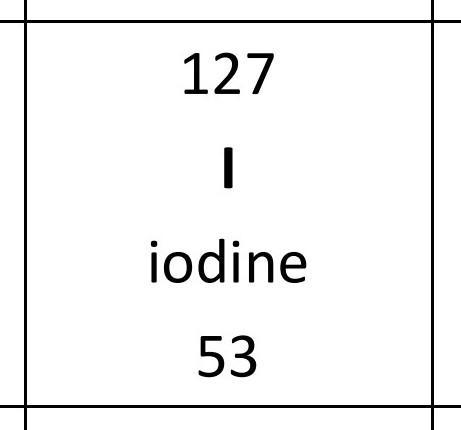
What process results in a new substance being formed?
Chemical reaction
Physical change
Mixing
Answers
Answer:
Chemical reaction
Explanation:
Write 3 equations that is:
Metal + Acid (ex: dense H2SO4, HNO3) -> salt + H2O + (NO2/ NO/ SO2/...)
ex: Cu + 2H2SO4 (dense) -> CuSO4 + 2H2O + SO2
Answers
Here are three equations representing the chemical reaction between a metal and an acid:
Zinc + 2HCl → ZnCl2 + H2
Iron + 2HNO3 → Fe(NO3)2 + H2O + NO
Magnesium + 2H2SO4 → MgSO4 + 2H2O + SO2
The three equations representing the reaction :
Zinc + 2HCl → ZnCl2 + H2In this reaction, zinc (metal) reacts with hydrochloric acid to form zinc chloride and hydrogen gas.
Iron + 2HNO3 → Fe(NO3)2 + H2O + NOIn this reaction, iron (metal) reacts with nitric acid to form iron(II) nitrate, water, and nitric oxide.
Magnesium + 2H2SO4 → MgSO4 + 2H2O + SO2In this reaction, magnesium (metal) reacts with sulfuric acid to form magnesium sulfate, water, and sulfur dioxide.
In each of these equations, the metal reacts with the acid to produce a salt, water, and sometimes additional products such as hydrogen gas (H2), nitrogen dioxide (NO2), nitrogen monoxide (NO), or sulfur dioxide (SO2), depending on the specific acid and reaction conditions.
For more such questions on chemical reaction
https://brainly.com/question/14106530
#SPJ8
when you put water into the freezer of your refrigerator, it enters a state of less molecular disorder when it freezes. explain why or why not this is an exception to the principle of entropy.
Answers
When water freezes, it undergoes a transition from a disordered state (liquid) to an ordered state (solid). This may seem like it contradicts the principle of entropy, which states that the entropy of a closed system will tend to increase over time.
However, the principle of entropy applies to the entire system, including the surroundings. When water freezes in the freezer of a refrigerator, the energy released by the water is absorbed by the surroundings, such as the air in the freezer. This leads to an increase in entropy in the surroundings, which compensates for the decrease in entropy in the water.
In other words, the decrease in molecular disorder in the water is offset by an increase in disorder in the surroundings. This means that the principle of entropy still holds true, even though the water is entering a state of less molecular disorder when it freezes.
To know more about entropy here
https://brainly.com/question/22145368
#SPJ4
A compound with an empirical formula of C2H3Br2 has a molar mass of 373.69 g/mol. What is the molecular formula
Answers
The molecular formula of the compound is C4H6Br4 of a compound whose empirical formula is C2H3Br2 and has a molar mass of 373.69 g/mol.
Empirical formula: The empirical formula is the smallest whole number ratio of the elements present in a compound. On the other hand, the molecular formula is the actual formula of the compound that indicates the exact number of each type of atom in a molecule. The empirical formula is usually written first, followed by the molecular formula.
Molar mass: The molar mass of a compound is the mass of one mole of that compound. The molar mass of a compound is calculated by adding up the atomic masses of the elements present in the compound. The unit for molar mass is grams per mole (g/mol).
Given, empirical formula of the compound = C2H3Br2, molar mass = 373.69 g/mol.
The molar mass of the empirical formula can be calculated as follows:
Molar mass of C2H3Br2 = (2 x atomic mass of C) + (3 x atomic mass of H) + (2 x atomic mass of Br)
= (2 x 12.01 g/mol) + (3 x 1.01 g/mol) + (2 x 79.90 g/mol)
= 24.02 g/mol + 3.03 g/mol + 159.80 g/mol
= 186.85 g/mol
We know that the molecular formula of the compound is a multiple of the empirical formula. So, the molecular formula can be written as follows:
Molecular formula = n x empirical formula
where n is a whole number.
The ratio of molar mass to empirical formula mass can be used to determine the value of n.
n = molar mass / empirical formula mass
n = 373.69 g/mol / 186.85 g/mol
n = 2
Therefore, the molecular formula of the compound is twice the empirical formula. Hence, the molecular formula of the compound is C4H6Br4.
More on empirical formula : https://brainly.com/question/1603500
#SPJ11
2. Which statement is TRUE about ionic compounds?
A. In an ionic compound, the positive ion is the cation, the negative ion is the anion, and the cation
is always named first.
B. In an ionic compound, the positive ion is the cation, the negative ion is the anion, and the anion
is always named first.
C. In an ionic compound, the positive ion is the anion, the negative ion is the cation, and the anion
is always named first.
D. In an ionic compound, the positive ion is the anion, the negative ion is the cation, and the cation
is always named first.
Answers
Answer:
I think it's c I hope this helps
What number of cations is present in 1.17g of sodium chloride ?
Answers
Answer:
1.2 × 10^22 atoms.
Explanation:
Firstly, cations refers to the positively charged atom in the ionic compound, which is Na+.
Given the mass of NaCl as 1.17g, the number of moles of NaCl can be calculated this:
Molar mass of NaCl = 23 + 35.5
= 58.5g/mol
Mole = mass/molar mass
Mole = 1.17/58.5
Mole = 0.02moles
Using Avagadro's number, 6.022 × 10^23 atoms of Na+ are in 1 mole of NaCl.
In 0.02 moles of NaCl, there are 0.02 × 6.022 × 10^23 of Na+
0.1204 × 10^23 atoms
1.2 × 10^22 atoms of Na+ (cation)
Cobalt is an element with the formula, Co. How do you know cobalt is an element?
Answers
Answer:
It is in the periodic table, group 9 with the atomic number 7
it's a pure substance consisting only of atoms that all have the same numbers of protons in their nuclei.
PLSSS HELP. And there’s also at the bottom of the picture another option if u didn’t see it

Answers
Answer:
B. Gradualism
Explanation:
-) Potassium is also in Group 1 of the periodic table. Potassium reacts with water in a similar
way to lithium. Write down two differences you would see between the reactions of
potassium and lithium with water.
Answers
Lithium react slowly with water while potassium react violently with water. potassium caught fire while lithium does not caught fire.
What is chemical reaction?Chemical reaction is defined as a procedure in which one or more compounds, known as reactants, are changed into one or more distinct substances, known as products.
It can also be defined as a process in which two or more molecules collide with the proper orientation and enough force to produce a new product.
There are mainly five types of reaction.
Synthesis reactionAcid base reactionRedox reactionDecomposition reactionPrecipitation reactionThus, Lithium react slowly with water while potassium react violently with water. potassium caught fire while lithium does not caught fire.
To learn more about chemical reaction, refer to the link below:
https://brainly.com/question/3461108
#SPJ1
Ten plants are grown in equal amounts of sunlight with equal amounts of water and varying amounts of fertilizer.
Fertilizer is a(n):
Answers
which amine has the following spectroscopic data? 1h nmr: 1.15 ppm (9h singlet), 1.41 ppm (2h broad singlet) 13c nmr: 32.6 ppm, 47.4 ppm
Answers
The amine that has the given spectroscopic data is N,N-dimethylisopropylamine. To identify the amine, we will analyze the provided 1H NMR and 13C NMR data.
1H NMR:
- 1.15 ppm (9H singlet): This indicates three methyl groups (\(CH_{3}\)) present in the molecule, each contributing three protons.
- 1.41 ppm (2H broad singlet): This indicates a methylene group (\(CH_{2}\)) attached to a nitrogen atom (amine group).
13C NMR:
- 32.6 ppm: This corresponds to a carbon that is attached to the nitrogen atom (amine) and is part of the methylene group (\(CH_{2}-N\)).
- 47.4 ppm: This corresponds to the carbon that has three methyl groups (\(CH_{3}\)) attached to it.
Taking these pieces of information together, we can deduce the structure of the amine: N,N-dimethylisopropylamine. This molecule has one \(CH_{2}-N\) group, with the nitrogen atom bonded to two methyl groups and a central carbon atom connected to three methyl groups.
Based on the provided 1H NMR and 13C NMR spectroscopic data, the amine in question is N,N-dimethylisopropylamine.
For more information on NMR spectroscopy kindly visit to
https://brainly.com/question/31594990
#SPJ11
what is true if ln k (natural log of the equilibrium constant) is positive
Answers
If the natural logarithm of the equilibrium constant (ln K) is positive, it indicates that the equilibrium constant (K) is greater than 1. This means that the forward reaction is favored, and the concentration of products is higher compared to the reactants at equilibrium.
The equilibrium constant (K) is a numerical value that describes the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction. The natural logarithm of K, represented as ln K, is a mathematical operation that helps to interpret the magnitude and direction of the equilibrium constant.
When ln K is positive, it implies that K itself is greater than 1. In other words, the concentration of products is larger than that of reactants at equilibrium. This indicates that the forward reaction is favored and that the system has a higher tendency to proceed in the forward direction, resulting in a relatively higher concentration of products.
The positive value of ln K implies that the forward reaction is spontaneous and thermodynamically favorable. The larger the positive value of ln K, the greater the concentration of products compared to the reactants at equilibrium, indicating a stronger bias towards the forward reaction. Conversely, if ln K were negative, it would suggest that the equilibrium constant is less than 1, indicating a higher concentration of reactants and a backward reaction favored at equilibrium.
To learn more about equilibrium constant click here:
brainly.com/question/31491716
#SPJ11
the shrinking of a strawberry from water loss after being sprinkled with sugar is an example of
Answers
The shrinking of a strawberry from water loss after being sprinkled with sugar is an example of osmosis.
Osmosis is the movement of water from an area of high water concentration to an area of low water concentration. When sugar is sprinkled on a strawberry, the sugar molecules draw water out of the strawberry cells. This causes the strawberry to shrink.
Osmosis is a common process that occurs in living cells. It is important for the transport of nutrients and waste products in cells. Osmosis can also be used to preserve food.
For example, fruits and vegetables can be preserved by soaking them in a solution of sugar and water. The sugar solution will draw water out of the cells of the fruit or vegetable, making it difficult for bacteria to grow.
To know more about osmosis, refer here:
https://brainly.com/question/31028904#
#SPJ11
What is not required to determine the amount of heat that must be added for water to steam?
Answers
The amount of heat that must be added for water to steam changes in
H (fusion).
What must be added to increase the temperature of steam?Water exists as a gas in steam. A sufficient amount of heat must be applied to the water to get it to the boiling point, and then further heat must be applied to transform the water's condition from liquid to steam without raising its temperature.
In saturated steam, the connection between temperature and pressure is strictly proportional. In other words, pressure rises as the temperature does.
The amount of heat or energy needed to raise a substance's temperature by 1 °C per unit mass of that substance is known as its specific heat capacity. The equation is Cv = Q / (T m).
The change in heat required to turn water into steam is measured in H (fusion).
To learn more about fusion refer to:
https://brainly.com/question/17870368
#SPJ4
g based on the semi-structural formulas of the three compounds listed above, identify the corresponding lewis structure:
Answers
The three compounds mentioned in the problem are not listed. Please provide the names or formulas of the compounds for a proper answer.What is Lewis structure?Lewis structures, also known as electron dot structures, are diagrams that depict valence electrons between atoms in a molecule.
To symbolize the valence electrons that surround an atom, Lewis structures employ dots. A shared pair of electrons is indicated by a line drawn between two atoms. Lewis diagrams depict the bonding and unshared electrons of a molecule or an ion in a way that suggests the molecule or ion's three-dimensional geometry.
The octet rule applies to the majority of molecules, which means that each atom in a molecule must have eight valence electrons (an octet) in its outer shell.
Learn more about compounds:
https://brainly.com/question/26487468
#SPJ11
What kinds of attractive forces may exist between particles in molecular crystals?.
Answers
The attractive forces that may exist between particles in molecular crystals are dipole-dipole forces; hydrogen bonding; London dispersion forces.
Molecular crystals are substances that have highly vulnerable intermolecular binding, which includes dry ice (solidified carbon dioxide), solid varieties of noble gases, and crystals of several natural compounds. Diamond is the purest form of carbon in which each carbon atom is covalently bonded to 4 different carbon atoms. as a result diamond is an instance of a covalent crystal.
Dipole-dipole forces are appealing forces between the positive end of 1 polar molecule and the negative end of every other polar molecule. Dipole-dipole interactions occur while the partial expenses fashioned within one molecule are interested in a contrary partial price in a close-by molecule. Polar molecules align in order that the high-quality quit of 1 molecule interacts with the negative cease of every other molecule.
Learn more about molecular crystals here:-https://brainly.com/question/1325088
#SPJ4
how can teh human body looose heat? (conduction, convecion, radiation) and WHY
Answers
Answer:
Radiation and conduction
Explanation:
The human body lose heat by these two ways, because everything that touches our bodies and is in a temperature below us will receive part of our heat (conduction), and you can figure out that we lose heat by radiation because a human bright in a heat cam