Answers
Answer: Number of atoms of Fe = 5.214x10²²atoms
Explanation:
First, we'll calculate the number of moles of Fe using the given number of atoms:
Number of moles = Number of atoms / Avogadro's number-----------(i)
Number of atoms of Fe = 5.22x10²²atoms
Avogadro's number = 6.022x10²³atoms/mol)
now putting values in equation (i)
Number of moles = 5.22x10²²atoms ₓ 6.022x10²³atoms/mol)
Number of moles of Fe = 0.0866 moles
To find the number of atoms of Fe, we can use Avogadro's number again:
Number of moles = Number of atoms / Avogadro's number-----------(i)
Number of moles of Fe = 0.0866 moles
Avogadro's number = (6.022x10²³atoms/mol)
Number of atoms of Fe = 5.214x10²²atoms
Related Questions
oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
How many liters of carbon dioxide can be produced if 37.8 grams of carbon disulfide react with excess oxygen gas at 28.85 degrees Celsius and 1.02 atmospheres?
CS2(l) + 3O2(g) yields CO2(g) + 2SO2(g)
2.78 liters
5.95 liters
12.1 liters
11.9 liters
Answers
The volume of carbon dioxide produced is approximately (d) 11.9 liters.
To determine the amount of carbon dioxide (C\(O_2\)) produced when 37.8 grams of carbon disulfide (C\(S_2\)) reacts with excess oxygen gas (\(O_2\)), we need to use stoichiometry and the given balanced chemical equation:
C\(S_2\)(l) + 3\(O_2\)(g) → C\(O_2\)(g) + 2S\(O_2\)(g)
First, we calculate the number of moles of C\(S_2\) using its molar mass:
Molar mass of (C\(S_2\)) = 12.01 g/mol (C) + 32.07 g/mol (S) × 2 = 76.14 g/mol
Number of moles of (C\(S_2\)) = mass / molar mass = 37.8 g / 76.14 g/mol ≈ 0.496 mol
From the balanced equation, we can see that the stoichiometric ratio between (C\(S_2\)) and C\(O_2\) is 1:1. Therefore, the number of moles of C\(O_2\) produced will also be 0.496 mol.
Now we can use the ideal gas law to calculate the volume of C\(O_2\) at the given temperature and pressure. The ideal gas law equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Converting the temperature from Celsius to Kelvin:
T(K) = 28.85°C + 273.15 = 302 K
Using the ideal gas law:
V = nRT / P = (0.496 mol) × (0.0821 L·atm/mol·K) × (302 K) / (1.02 atm) ≈ 11.9 L
The correct answer is 11.9 liters.
for more questions on carbon dioxide
https://brainly.com/question/26150306
#SPJ8
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
2. How can you change the concentration of a solution, yet keep the number of moles of solute the same
Answers
One can change the concentration of a solution while keeping the number of moles of solute the same by reducing the volume of solvent in the solution.
What is molarity?The molarity of a solution is the number of moles of solute per unit volume of solvent.
The number of moles of solute in a unit volume of solvent can be kept constant while the volume of the solvent reduced in order to increase the concentration of a solution.
For example, a solution with 2 moles of solute in 1 L of solvent will have a molarity of 2 M.
If the amount of solvent is reduced to 0.5 L, the molarity will become:
2/0.5 = 4 M
More on molarity can be found here: https://brainly.com/question/12127540
what keeps molecules together in a liquid and stops them from becoming a gas?
Answers
Answer:
intermolecular interactions
Explanation:
hope this helps
The intermolecular attraction keeps molecules together in a liquid and stops them from becoming a gas.
What is intermolecular attraction ?The electromagnetic forces of attraction and repulsion that act between atoms and other types of nearby particles, such as atoms or ions, are examples of intermolecular forces.
Liquids have qualities that are halfway between gases and solids, yet they are closer to solids. Intermolecular forces, in opposed to intramolecular forces like covalent ties that bind atoms together in molecules as well as polyatomic ions, keep molecules with each other in a liquid or solid.
To know more about intermolecular force click here.
https://brainly.com/question/9007693.
#SPJ2
Please check the attached. Fairly easy question.

Answers
To encourage more product, CS₂, to form from the given reaction add more H₂S, hence option A is correct.
Equilibrium exists when the reactants and products are in balance. The concentration varies with each stop. A reaction takes place and a product is produced when two reactants are mixed. The concentration of the product rises as the reactant concentration falls.
One method is to change the reactant or product of a chemical process when it reaches equilibrium.
The equilibrium changes as more reactant is introduced, producing more product as a result. To lessen the stress, adding more product causes the equilibrium to switch to reactants.
Learn more about reactions, here:
https://brainly.com/question/16737295
#SPJ1
Which of the following is used to keep track of the heat flow inside a calorimeter?
Answers
Any time a liquid loses or receives energy, the temperature of the liquid changes.To calculate the amount pf energy change, the calorimeter monitors the weight of the fluid as well as the temperature change.
In a calorimeter, what thermometer is used?The 6000 series calorimeters' control systems serve as the foundation for the 6772 Calorimetric Thermometer, a high precision temperatures measuring device.It is a crucial component of both the 6755 Solution Calorimeter and the 6725 Semi-micro Calorimeter.
What equipment is utilized in calorimetry?The calorimeter is a piece of equipment used in calorimetry, a procedure for calculating heat capacity and measuring the temperature of chemical processes or other physical changes.Among the most popular kinds are differential scanning operating, thermal micro cathode rays, titration calorimeters, and accelerated rate calorimeters.
To know more about energy visit:
https://brainly.com/question/1932868
#SPJ1
What conclusion can you make about the hardness of the mineral?
Answers
Answer:
Glass is used to help determine the hardness of a mineral.
Explanation:
hope this helps
It can be concluded that hardness of the mineral is due to the metal content present in it in the form of ore as it is a characteristic of metals to be hard.
What are minerals?Minerals are defined as a chemical compound which has a well -defined composition and possesses a specific crystal structure.It occurs naturally in the pure form.
If a compound occurs naturally in different crystal structure then each structure is considered as a different mineral.The chemical composition of a mineral varies depending on the presence of small impurities which are present in small quantities.
Some minerals can have variable proportions of two or more chemical elements which occupy equivalent position in the crystal structure.It may also have variable composition which is split into separate species.
Physical properties of minerals include color,streak, luster,specific gravity and cleavage.
Learn more about minerals,here:
https://brainly.com/question/1333886
#SPJ2
what are physical properties of matter
Answers
Answer: The physical properties are mass, weight, volume, and density.
Explanation:
. ¿Cuantos moles de Fosfato de Bario se producen cuando reaccionan 0.38 mol de Nitrato
de bario? Suponga que el ácido fosfórico está en exceso. Base sus cálculos en la siguiente
ecuación.
Ba(NO3)2(aq) + HAPO.(aq)
Ba,(PO.),(s) + HNO3(aq)
-
Answers
Respuesta:
0.13 mol
Explicación:
Paso 1: Escribir la ecuación química balanceada
3 Ba(NO₃)₂ + 2 H₃PO₄ ⇒ Ba₃(PO₄)₂ + 3 H₂O
Paso 2: Establecer la relación molar apropiada
La relación molar de Ba(NO₃)₂ a Ba₃(PO₄)₂ es 3:1.
Paso 3: Calcular cuantos moles de fosfato de bario se producen a partir de 0.38 moles de nitrato de bario
0.38 mol Ba(NO₃)₂ × 1 mol Ba₃(PO₄)₂/3 mol Ba(NO₃)₂ = 0.13 mol Ba₃(PO₄)₂
can anyone heelp me plzzz

Answers
Answer: 1
Explanation: C= 1 if you round 1.04 to a single digit the answer is 1
Why in strong or weak acid solutions, autoionization of water produces less H3O+ amounts than in pure water?
Answers
In strong or weak acid solutions, autoionization of water produces less H3O+ amounts than in pure water as according to le Ch atelier principle, addition of H3O+ causes the H2O equilibrium to shift left.
The awareness of hydronium could be very tons better than a susceptible acid due to the fact robust undergoes entire dissociation and a susceptible acid undergoes best partial dissociation generating lesser quantity of Hydronium ions. Adding an acid to water consequently has an impact at the awareness of each the H3O+ and OH- ions. Because it's miles a supply of this ion, including an acid to water will increase the awareness of the H3O+ ion. Adding an acid to water, however, decreases the quantity to which water dissociates.
To learn more about partial dissociation check the link below-
https://brainly.com/question/13923449
#SPJ4
If a steam reforming line processes methane at a temperature of
725.0°C and a pressure of 12.00 atm, what is the volume (in L) that
20.0 moles of methane would occupy in the reactor?
Answers
Answer:
ideal gas law. PV=nRT
Explanation:
pressure x volume = #moles x R (constant of 8.31) x temp
Calculate the mass of sucrose necessary to make a 5% by mass sucrose solution if the solution contains 50.0 ml of distilled water. (Hint: the total mass of the solution is the mass of sucrose plus the mass of water.)
Answers
Answer:
2.6 g
Explanation:
Percent concentration = 5%
Volume of water = 50ml
Since 1ml of water = 1g of water
Mass of water= 50g
Let the mass of sucrose be x
Mass% = mass of solute/mass of solute + mass of solvent × 100
5= x/x+ 50 ×100
5/100 = x/x + 50
0.05 = x/x + 50
0.05(x + 50) = x
0.05x + 2.5 = x
2.5 = x - 0.05x
2.5 = 0.95x
x= 2.5/0.95
x=2.6 g
What is the theoretical yield of BOTH products when 100.0 g of Cr2O3 reacts with 100.0 g of C?
Answers
Theoretical yield of Cr is 68.43 g and the theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
What is theoretical yield?Quantity of a product obtained from complete conversion of the limiting reactant in chemical reaction is called theoretical yield.
2 Cr2O3 + 3 C → 4 Cr + 3 CO2
Molar mass of Cr2O3 is 152 g/mol, and the molar mass of C is 12.01 g/mol. Therefore, 100.0 g of each reactant is equivalent to:
100.0 g Cr2O3 / 152 g/mol = 0.6579 mol Cr2O3
100.0 g C / 12.01 g/mol = 8.329 mol C
The stoichiometry of the balanced equation tells us that for every 2 moles of Cr2O3, we need 3 moles of C. Therefore, if we have 0.6579 mol of Cr2O3, we need (3/2) x 0.6579 = 0.9868 mol of C to react completely. However, we only have 8.329 mol of C, which is in excess. This means that Cr2O3 is limiting reactant, and C is excess reactant.
From the balanced equation, 2 moles of Cr2O3 produce 4 moles of Cr.
Therefore, 0.6579 mol of Cr2O3 will produce (4/2) x 0.6579 = 1.316 mol of Cr.
Molar mass of Cr is 52.0 g/mol, so the theoretical yield of Cr is 1.316 mol x 52.0 g/mol = 68.43 g.
Therefore, 8.329 mol of C will produce (3/1) x 8.329 = 24.99 mol of CO2.
Molar mass of CO2 is 44.01 g/mol, so the theoretical yield of CO2 is 24.99 mol x 44.01 g/mol = 1099.25 g or 1.1 kg.
Therefore, theoretical yield of Cr is 68.43 g and theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
To know more about theoretical yield, refer
https://brainly.com/question/25996347
#SPJ1
Calculate the molarity of a solution prepared by diluting 165 mL of
0.700 M calcium chloride to 900.0 mL.
Answers
Answer:
The product of transcription is RNA, which can be encountered in the form mRNA, tRNA or rRNA while the product of translation is a polypeptide amino acid chain, which forms a protein.
Explanation:
Describe the cause and effect relationship between density and ocean currents.
Answers
Answer:
Differences in water density affect vertical ocean currents. Denser water tends to sink, while less dense water tends to rise. Other causes of currents include tides, rain, runoff, and ocean bottom topography. Topography is the surface features of a place. Ocean topography includes slopes, ridges, valleys, and mountains! All these things are found at the bottom of the ocean, and can influence currents.
The cause-and-effect relationship between density and ocean currents is the mixing and circulation are influenced by the density differences between the various layers of the water column.
What are ocean currents?The continuous, predictable, and directional movement of seawater known as ocean currents is caused by gravity and wind.
Ocean vertical currents are influenced by variations in water density. Less dense water tends to rise, while denser water sinks. Tides, rainfall, runoff, and the topography of the ocean bottom are additional causes of currents.
Thus, the mixing and circulation are influenced by the differences in densities between the various layers of the water column, which is the cause-and-effect relationship between density and ocean currents.
To learn more about ocean currents, refer to the below link:
https://brainly.com/question/1543125
#SPJ2
Please I’m skipping math and Ela for this help

Answers
Answer:
c
Explanation:
because it is c ok ????????????
Which of the following is NOT true about one mole?
the number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron
O 12 g of carbon equals one mole of carbon atoms
the mass of 1 mole of carbon atoms equals the mass of 1 mole of boron atoms
one mole contains 6.02 x 10
23
particles
Answers
Mass=molesx molar mass
And the molar masses of these two elements are different so their masses aren’t equal
The number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron is false because the molar mass of carbon is different from that of boron. Option A is correct.
The number of moles of an element is expressed as the ratio of the mass of the substance to its molar mass.Moles = Mass/Molar massThis shows that the number of moles of the substance is dependent on the molar mass of the substance.
From the listed option the number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron is false because the molar mass of carbon is different from that of boron
Learn more on moles here: https://brainly.com/question/24256751
How many revolutions does the hour hand on a clock make in a year using dimensional analysis for high school
Answers
A hereditary mutation is one that is unique to the individual organism?
True or false
Answers
Answer:
I think it is True
Explanation:
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according to
the equation:
H3PO4(aq) + 3NaOH(aq) + Na3PO4(aq) + 3H2O(aq)
A) O 0.05 L
B) O6.93 L
C) O0.44 L
D) 03.01 L
E) 436.43 L

Answers
Taking into account the definition of molarity and the stoichiometry of the reaction, the correct option is option C) 0.44 L of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid.
The balanced reaction is:
H₃PO₄ (aq) + 3 NaOH (aq) → Na₃PO₄ (aq) + 3 H₂O(aq)
Then, by stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₃PO₄: 1 mole NaOH: 3 moles Na₃PO₄: 1 mole H₂O: 3 molesMolarity is the number of moles of solute that are dissolved in a given volume.
Molarity is determined by:
\(Molarity=\frac{number of moles of solute}{volume}\)
Molarity is expressed in units \(\frac{moles}{liter}\).
In this case, 0.42 L of 2.39 M phosphoric acid reacts. So, by definition of molarity, the number of moles that participate in the reaction is calculated as:
\(2.39 \frac{moles}{liter}=\frac{number of moles of phosphiric acid}{0.42 liters}\)
Solving:
number of moles of phosphiric acid= 2.39 \(\frac{moles}{liter}\)* 0.42 liters
number of moles of phosphiric acid= 1.0038 moles ≅ 1 mole
Approaching 1 mole of the amount of phosphoric acid required, then by stoichiometry of the reaction, 3 moles of NaOH are necessary to react with 1 mole of the acid.
Then by definition of molarity and knowing that 6.9 M NaOH is needed, you can calculate the necessary volume amount of NaOH by:
\(6.9 \frac{moles}{liter} =\frac{3 moles}{volume}\)
Solving:
6.9 \(\frac{moles}{liter}\)* volume= 3 moles
\(volume=\frac{3 moles}{6.9\frac{moles}{liter} }\)
volume= 0.44 L
The correct option is option C) 0.44 L of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid.
Learn more about molarity with this example: https://brainly.com/question/15406534?referrer=searchResults
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
on June 21st the rays of the sun fall directly on the Tropic of Cancer true or false pls tell the ans and I follow u
Answers
Answer:
True
Explanation:
it does! because of the angle between Saturn and Earth and the Sun.
Hope this helps plz hit the crown :D
Which combination makes up most of the mass of an atom?
A. Electron and proton
B. Electron and neutron
C. Proton and neutron
D. None of these make up atom’s mass
Answers
Answer:
Explanation:
its c. protons and neutron
I just need 1 more answer for "products" and I'm done!

Answers
The product would be aluminum oxide, \(Al_2O_3\).
Word equationThe word equation for a reaction can be interpreted as the formula equation of the same reaction by writing out the chemical formulas of the reactants and products accordingly.
Now, let's consider the reaction in question:
Four atoms of aluminum metal react with three molecules of oxygen to produce two molecules of aluminum oxide.
Four atoms of aluminum = 4 Al
Three molecules of oxygen = \(3O_2\)
two molecules of aluminum oxide = \(2Al_2O_3\)
Thus, the formula equation would be: \(4Al + 3O_2 --- > 2Al_2O_3\)
In other words, the product would be \(Al_2O_3\).
More on word equations can be found here: https://brainly.com/question/15423243
#SPJ1
the human population grew form 1 billion in the year 1800to blank billion in the year 200
Answers
The human population grew from 1 billion in the year 1800 to approximately 7.8 billion in the year 2021.
In the year 1800, the estimated global human population was around 1 billion. Over the next two centuries, significant advancements in technology, medicine, agriculture, and improved living conditions contributed to a rapid increase in population.
The growth rate of the human population began to accelerate in the 20th century. By the year 1927, the global population reached 2 billion. It took just 33 years for the population to double, reaching 4 billion in 1960. The population continued to grow at an unprecedented rate, with 6 billion people on Earth by the year 1999. As of 2021, the estimated global population stands at approximately 7.8 billion.
This remarkable growth in population can be attributed to several factors, including advancements in healthcare leading to reduced infant mortality rates, improved access to education and contraception, increased agricultural productivity, and overall socio-economic development.
It's important to note that population growth has not been uniform across all regions. Different countries and regions have experienced varying rates of population growth due to factors such as fertility rates, mortality rates, migration patterns, and government policies.
For more such questions on population visit:
https://brainly.com/question/30148263
#SPJ8
The mass of the products will decrease as they absorb heat energy.
The mass of the products will increase as they absorb heat energy.
The total mass of the products will decrease as the volume increases.
The total mass of the products will equal the total mass of the reactants.
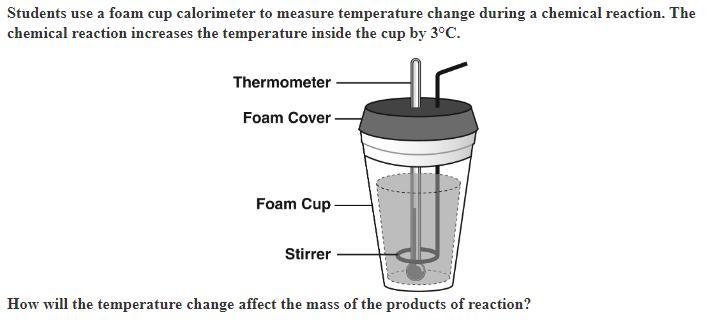
Answers
The total mass of the products will equal the total mass of the reactants.
What is the calorimeter?A calorimeter is a device used to measure the heat of chemical reactions or physical changes. It works by measuring the heat absorbed or released during a reaction or process by monitoring changes in temperature.
Calorimeters can be used to study a wide range of chemical reactions, including combustion, acid-base reactions, and reactions involving enzymes.
Calorimetry is an important technique in the study of thermodynamics, and it has many practical applications.
Learn more about calorimeter:https://brainly.com/question/28034251
#SPJ1
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of 0.270 A is passed through an electroplating cell with an aqueous solution of Ag_2 SO_4 in the cathode compartment for 72.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Mass of the pure silver deposited on a metal object made into the cathode of the cell is calculated to be 0.0217 gm.
What is electroplating?The process of using electrodeposition to coat an object in a layer of metal is called electroplating .
As we know that, Q = I * t
=0.270 * 72
= 19.44 C
Here Q is quantity of electricity , I is current in amperes = 0.270 A (given)
t is time in seconds (72.0 sec)
As 96500 Coulomb of electricity electrolyzes 1 mole of Ag
then,19.44 C of electricity deposits,
=1/96500 * 19.44
= 0.000201 moles of Ag
Mass of Ag is = number of moles * molar mass
= 0.000201 * 108
= 0.0217 gm
Thus, mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0217 gm.
To know more about electroplating, refer
https://brainly.com/question/16266707
#SPJ4
1. Explain how you would determine the enthalpy of reaction for the hypothetical reaction A2X4(l) + X2(g) → 2AX3(g) using the following information. You do not need to calculate an answer. Respond to the prompt with a minimum response length of 50 words.

Answers
we can determine the enthalpy of reaction for the hypothetical reaction A2X4(l) + X2(g) → 2AX3(g) using the following steps:
write the balanced chemical equation for the reactionwe obtain the standard enthalpies of formation for each compoundwe apply Hess's law calculate the enthalpy of reactionwe then add up the changes to get the total enthalpy change for the reaction State Hess law?Hess's Law of Constant Heat Summation states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes.
The law is Hess's Law of Constant Heat Summation is described as a manifestation that enthalpy is a state function.
Learn more about Hess law at:
https://brainly.com/question/9530080
#SPJ1