Mercury, the only metal that is liquid at room temperature, has a density of 13.6g/cm^3. How much does 25cm^3 of mercury weigh?
Answers
Answer:
The mercury with volume of 25 cm³ having weight of 340 g.
Explanation:
Given data:
Density of mercury = 13.6 g/cm³
Volume of mercury = 25 cm³
Mass of mercury = ?
Solution:
We will apply density formula.
d = m/v
13.6 g/cm³ = m/ 25 cm³
m = 13.6 g/cm³ × 25 cm³
m = 340 g
The mercury with volume of 25 cm³ having weight of 340 g.
Related Questions
how many times acidic is pOH 12 than pOH 10
Answers
Answer:
100x less acidic
Explanation:
pOH is the inverse of pH, which is a measure of the acidity or basicity of a solution. The lower the pH of a solution, the more acidic it is, and the higher the pOH, the less acidic it is.
To determine how many times more acidic a solution with a pOH of 12 is compared to a solution with a pOH of 10, we can use the formula:
pH1 / pH2 = 10^(pOH1 - pOH2)
In this case, we are given that the pOH of the first solution is 12 and the pOH of the second solution is 10. We can plug these values into the formula to calculate the ratio of acidity:
pH1 / pH2 = 10^(12 - 10)
= 10^2
= 100
Therefore, a solution with a pOH of 12 is 100 times less acidic than a solution with a pOH of 10.
a student draws the orbital diagram below for the 3p electrons in an s atom. what, if anything, is incorrect about the drawing?
Answers
The filling up of electrons in atomic orbitals is governed by three rules. An orbital diagram is represented on the basis of the above three rules.
What is orbital diagram?The pictorial representation of the electrons present in an atom is given by the orbital diagrams. The rules which are required in forming the orbital diagrams are Aufbau principle, Hund's rule and Pauli's exclusion principle.
According to Aufbau principle, each electron occupies the lowest energy orbital, only two electrons fit into a single orbital is Pauli's exclusion principle and electrons go into different orbitals in the same sub level before doubling.
The orbital diagram of 3p electrons of 'S' atom is attached below.
To know more about orbital diagram, visit;
https://brainly.com/question/11270343
#SPJ1
1. In this lab, you will be tasked with identifying various elements, based on the first-hand observation of their physical properties. For each of the following elements, perform on-line research to predict what the color, texture, and lustre of the substance will be:
Solid magnesium
Solid carbon (graphite)
Solid zinc
Answers
Solid magnesium: The color of solid magnesium is silver-white, it has a smooth texture, and a metallic lustre.
Solid carbon (graphite): The color of solid carbon (graphite) is black, it has a soft and slippery texture, and a dull lustre.
Solid zinc: The color of solid zinc is bluish-white, it has a relatively smooth texture, and a metallic lustre.
What is lustre?
Lustre is a term used to describe the way that light interacts with the surface of a material, specifically in terms of its reflective quality. It is a physical property of materials, and can be described as the appearance of the material's surface when it reflects light.
Materials can have different types of lustre, which are based on their reflective properties. For example, a material can have a metallic lustre, which is bright and reflective like a metal, or a vitreous lustre, which is shiny and glass-like. Other types of lustre include pearly, resinous, silky, and dull.
What is graphite?
Graphite is a naturally occurring form of the element carbon, and is one of the softest and most stable forms of carbon known. It is a crystalline allotrope of carbon, which means that it has a specific atomic structure that is different from other forms of carbon, such as diamond or amorphous carbon.
Graphite has a unique structure that consists of layers of carbon atoms arranged in a hexagonal lattice, which gives it a layered or sheet-like structure. Each layer is composed of carbon atoms that are strongly bonded together in a flat, two-dimensional arrangement. These layers are held together by weaker, non-covalent bonds, which allows them to slide over one another easily.
To know more about graphite, visit:
https://brainly.com/question/171380
#SPJ9
3. Calculate the number of atoms in a sample of carbon that has a mass of
276.23amu.
Answers
Answer:
1.39 x 10^25 atoms
Explanation:
12 amu of C has 6.02 x 10^23 atoms
so 276.23 amu has
(276.23)(6.02 x 10^23)/(12) = 1.39 x 10^25 atoms
HEAT ONLY FLOWS UPWARD; TRUE OR FALSE?
Answers
Answer:
it would be false. It doesn't only flow upward
Explanation:
which group is selected as secondary suffix if -OH and -CHO both are present in same compound
Answers
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula -OH and consists of an oxygen atom covalently bonded to a hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxyl groups. In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R-CH=O. The functional group itself is sometimes called an aldehyde, but it can also be classified as a formyl group. Aldehydes are ubiquitous and play an important role in technology and biology. The group priority is -OCH3>OH>COOH>CHO. According to the F. Cahn-Ingold-Prelog system, a priority (1, 2, 3, or 4) is assigned in descending order of atomic number to the atoms directly bonded to the stereocenter the atom with the highest atomic number gets the highest priority.
Match each atomic combination to the appropriate functional group.
- A. B. C. D. C-O-C
- A. B. C. D. O=C-O-H
- A. B. C. D. O=C-O-C
- A. B. C. D. C-O-H
A. Alcohol
B. Carboxylic acid
C. Ester
D. Ether
Answers
Answer:
The atomic combinations can be matched to the appropriate functional groups as follows:
- A. C-O-C: C. Ester
- B. O=C-O-H: B. Carboxylic acid
- C. O=C-O-C: C. Ester
- D. C-O-H: A. Alcohol
Explanation:
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
At a given temperature, 0.500 mols of CO and 1.50 moles of water vapor are added to a 2.50 L vessel. When the reaction reaches equilibrium, the [CO2] and [H2] are 0.00775 M. Find the [CO] and the [H2O] at equilibrium. Calculate the Keq and predict the sign of ΔG.
Answers
The concentrations of the reaction's reactants and products must be equal at equilibrium. Following is a description of how CO and H2O react to generate CO2 and H2: CO + H2O <=> CO2 + H2 We can determine the equilibrium CO and H2O concentrations using the available data.
The starting concentrations of CO and H2O are 0.800 M and 0.800 M, respectively, due to the total moles of CO and H2O being 2.00 moles and the total volume being 2.50 L. The equilibrium expression may be used to compute the equilibrium concentrations of CO and H2O: K = [CO2][H2]/[CO][H2O] K = (0.00775)(0.00775)/[CO] may be used to derive the equilibrium constant given that [CO2] and [H2] are both equal to 0.00775 M.
[H2O] K = (0.00775)(0.00775)/[0.0455], when the equilibrium concentrations of CO and H2O are plugged in.[0.0455]. ][0.0455] K = 0.0020 From this, we can calculate the equilibrium concentrations of CO and H2O: [CO] = 0.0455 M [H2O] = 0.0455 M .
The standard free energy change (G°), which can be calculated using the formula G° = -RTlnK, may be used to estimate the sign of G for this reaction. Since K > 1, we may anticipate a spontaneous response, meaning that G will be negative.
Learn more about concentrations at:
https://brainly.com/question/10725862
#SPJ1
If you travel east from 125 degrees west to 55 degrees east along the equator, how far will u travel in degrees
Answers
Answer:
180°
Explanation:
You are starting at 125° W and ending at 55° E.
It might be easier to find the distance travelled by using a number line.
Numbers west are negative and numbers east are positive.
Start at -125 and go in jumps of 10 until you reach +55.
You make 18 jumps of 10° each for a total of 180°.
You have travelled a distance of 180°.
What you have just done is equivalent to saying:
distance travelled = end - start = 55° - (-125°) = 55° + 125° = 180°

Which of the following describes a compound?
A.
a piece of pure carbon, containing only carbon atoms
B.
oxygen gas surrounding a solid piece of carbon
C.
a substance made of two oxygen atoms for each carbon atom
D.
carbon and oxygen atoms mixed without being bonded together
Answers
Answer:
Explanation:
D.
carbon and oxygen atoms mixed without being bonded together
How many molecules are in 1.2 moles of H₂O?
Answers
Explanation:
• A compound is a substance that is made by two or more atoms from different elements.
• A mole of a compound contains a number of molecules equivalent to Avogadro's number, 6.022 × 10^23.
• That is, one mole of a compound contains 6.022 × 10^23 molecules.
In this case we are given;
Number of moles of H2O as 1.2 moles
But, 1 mole of H2O contains 6.022 × 10^23 molecules.
We are required to calculate the number of molecules present;
• To calculate the number of molecules we are going to multiply the number of molecules in one mole by the number of moles.
• Therefore;
Number of molecules = 1.2 moles × 6.022 × 10^23 molecules/mole
= 7.226 × 10^23 molecules.
Thus, 1.2 moles of water contains 7.226 × 10^23 molecules.

For the molecule COBr2 Upload the correct Lewis structure (including resonance forms, if com to the PL What is the geometry of the molecule? Is it polar or non-polar? What is the hybridization of the central atom?
Answers
COBr2 is polar in nature and prefers to dissolve in polar solvents like water, ethanol, and methanol, among others, cobr2 is soluble in polar solvents.
Which way does Cobr2 polarize?Because cobr2 is polar in nature and prefers to dissolve in polar solvents like water, ethanol, and methanol, among others, cobr2 is soluble in polar solvents.Lewis structures use a 2D representation to show where the electrons are placed, whereas molecular geometry only uses 3 dimensions when the bond atoms are present (whether its lone-pair or atom).Given that COBr2 has a double bond, we anticipate that the Br-C-Br and Br-C-O angles will be only slightly above and below 120 degrees, respectively.To learn more about polar solvents refer to:
https://brainly.com/question/3184550
#SPJ4
PLEASE ANSWER NOW!!! This chart shows what happens when each object is placed on a balance with a 10 kg weight on the other side. Which statement is best supported by the information in the chart? Object 1 is the heaviest object. Object 3 is heavier than Object 1. The weight is heavier than Object 2. The weight is the heaviest object.

Answers
Answer:
Balanced forces do not cause a change in motion. When balanced forces act on an object at rest, the object will not move. ... Forces that cause a change in the motion of an object are unbalanced forces. Unbalanced forces are not equal and opposite.
Explanation:
Any push or pull is a force. To describe a force, you must know two things. You must know the size of the force and the direction of the force. Suppose two teams are playing tug of war. Each team is pulling with equal force, but in opposite directions. Neither team can make the other team move. Forces that are equal in size but opposite in direction are called balanced forces.
tug of war balanced
Balanced forces do not cause a change in motion. When balanced forces act on an object at rest, the object will not move. If you push against a wall, the wall pushes back with an equal but opposite force. Neither you nor the wall will move. Forces that cause a change in the motion of an object are unbalanced forces.
tug of war unbalanced 1
Unbalanced forces are not equal and opposite. Suppose that one of the teams in tug of war pulls harder than the other team. The forces would no longer be equal. One team would be able to pull the other team in the direction of the larger force.
Force and Motion
More than one force can act on an object at the same time. If you hold a paper clip near a magnet, you, the magnet and gravity all exert forces on the paper clip. The combination of all the forces acting on an object is the net force. When more than one force is acting on an object, the net force determines the motion of the object. In this example, the paper clip is not moving, so the net force is zero.
How do forces combine to form the net force? If the forces are in the same direction, they add together to form the net force. Suppose you and a friend are asked to move a piano for the music teacher. To do this, you pull on one end of the piano, and your friend pushes on the other end. Together, your forces add up to enough force to move the piano. This is because your forces are in the same direction. Because the forces are in the same direction, they can be added together to determine the net force. In this case, the net force is 45 N, which is plenty to move a piano - if it is on wheels, that is!
net force piano
If two forces are in opposite directions, then the net force is the difference between the two forces, and it is in the direction of the larger force. Consider two dogs playing tug of war with a short piece of rope. Each is exerting a force, but in opposite directions.
Notice below that the dog on the left is pulling with a force of 10 N, and the dog on the right is pulling with a force of 12 N. Which dog do you think will win the tug of war? Because the forces are in opposite directions, the net force is determined by subtracting the smaller force from the larger one. In this case, the net force is 2 N in the direction of the dog on the right. Give that dog a dog biscuit!
Answer:
A. Object 1 is the heaviest
Hope it works!
Explanation:
Help please chapter 7.1

Answers
2. 10N to the left
3. 0N they are balanced
4. 0N they are balanced
how to figure out how much a mole would weigh?
Answers
1)
2)
Types of Chemical Reaction Worksheet
A. Balance the reactions 1 to 6 and indicate which type of chemical reaction
(synthesis, decomposition, single-displacement, double- displacement or
combustion) is being represented:
3)
4)
5)
6)
―
C₂H₂ +
C₂H18 +
FeCl3 +
P+
HNO3 +
-
-
O₂ →
_0₂.
NaOH →
_ą₂ →
_H₂O + O₂ → H₂O₂
▬▬
2) Pb + FeSO,
CO₂ +
_P₂0₁
3) 2 BF, + 3 H₂O
CO₂ +
5) 2 Fe + O₂ +
H₂O
NaHCO3 → NaNO3 + H₂O + CO₂
H₂O
Fe(OH)3 +
NaCl
B. Identify the type of reaction as synthesis, decomposition,
single-replacement, double-replacement, and combustion:
1) Na,PO, + 3 KOH
→3 NaOH + K,PO,
PbSO, + Fe
- B₂0, + 6 HF
4) 2 AI + 6 HCI 2 AICI, + 3 H₂
Reaction Type:
Reaction Type:
Reaction Type:
2 H₂O2 Fe(OH),
Reaction Type:
Reaction Type:
Reaction Type:
the ele

Answers
A. Balanced reactions and reaction type is given below as asked in question above :
C₂H₂ + 5 O₂ → 4 CO₂ + 2 H₂O (combustion)
2 NaOH + Cl₂ → NaCl + NaClO + H₂O (double-displacement)
Fe + 2 HCl → FeCl₂ + H₂ (single-displacement)
P₄ + 5 O₂ → P₄O₁₀ (synthesis)
2 Fe + 2 NaOH + H₂O → 2 Fe(OH)₂ + 2 Na⁺ (double-displacement)
2 NaHCO₃ → 2 NaNO₃ + H₂O + 2 CO₂ (decomposition)
B. Reaction type:
3 Na₃PO₄ + K₃PO₄ → 6 NaOH + 2 K₃PO₄ (double-displacement)
PbSO₄ + Fe → Pb + FeSO₄ (single-displacement)
B₂O₃ + 6 HF → 2 BF₃ + 3 H₂O (double-displacement)
2 Al + 6 HCl → 2 AlCl₃ + 3 H₂ (single-displacement)
2 H₂O₂ → O₂ + 2 H₂O (decomposition)
Fe(OH)₃ + 3 NaCl → FeCl₃ + 3 NaOH (double-displacement)
To know more about double-displacement, visit:
https://brainly.com/question/29307794
#SPJ1
Which of the following compounds does NOT have a pH-dependent solubility?
a. Mg(OH)^2
b.Na2O
c. PbS
d. AgI
e.CaCo3
Answers
Sodium oxide \(Na_2O\) is a compound that does NOT have a pH-dependent solubility among the given options. Thus, option B is correct.
Sodium oxide is an ionic compound that is formed when the positive ions of the sodium cations combine with the negative ions of the oxide anions. When the Sodium oxide is dissolved in water, it will completely disassociate into sodium ions and hydroxide ions.
This disassociation of ions is purely independent of pH value. Because this reaction will not involve any proton or electron transfer. The solution is mainly determined by the ionic bond strength within the bond range and also based on the ability of water molecules to solvate the resulting ions.
To learn more about Sodium oxide
https://brainly.com/question/29068032
#SPJ4
PLEASE HELP EVERYONE KEEPS PUTTING LINKS STOP I'M NOT GONNA DOWNLOAD THAT

Answers
Answer:
they are? I think you should comment "STOP" to them
The picture below shows an open field with wildflowers.
Which of the following is NOT a way that this environment supports populations of bees?
Answers
Answer:
no picture you need add it
The atomic mall mass is an element depends upon the.
Answers
Answer:
Mass and relative abundance of each isotope of that element
The atomic mass of element has been dependent on mass of each isotopes and their abundance in nature.
The atomic mass has been the mass of neutrons and protons of the element. There has been the presence of the isotopes of the elements, with varying abundance, and varying atomic masses.
The atomic mass of the element has been the relative average atomic mass of the element with respect to the mass of the isotopes and their percent abundance.
The average atomic mass (amu) has been given by:
\(amu=M_1A_1\;+\;M_2A_2\)
Where, \(M_1A_1\) has been the atomic mass and abundance of isotope 1.
\(M_2A_2\) has been the atomic mass and abundance of isotope 2.
Thus, the average atomic mass has been dependent on mass of each isotopes and their abundance in nature.
For more information about the atomic mass, refer to the link:
https://brainly.com/question/5566317
Which property do the elements in each column of the representative elements series of the periodic table have in common?.
Answers
Answer: Valence electron
Explanation:
The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements.
What factors decrease the transfer of thermal energy?
ASAP
Answers
Answer:
What 3 factors influence the amount of thermal energy in an object, and how do they each affect it? Mass, temperature, and phase. More mass, more Thermal Energy. Higher temperature, more Thermal Energy.
ASAP=abbreviation. As soon as possible. 'fill in your form and send it to us ASAP' More example sentences.
what is the number of shells and number of valence electrons for the following elements: Na, O, I, Ca
Answers
The number of electrons present in the outermost shell is called valence electrons.
What are valence electrons?The number of electrons present in the last shell is called valence electrons. The atomic number of sodium is 11 and the atomic number of oxygen is 8.
There is 1 valence electron in sodium. The number of valence electrons in oxygen is 2.
The atomic number of iodine is 53. The number of the valence electron in iodine is 1. The atomic number of calcium is 20. The valence electron in calcium is 2.
Therefore, The number of electrons present in the outermost shell is called valence electrons.
To know more about valency, refer to the link:
https://brainly.com/question/12744547
#SPJ1
Question 5 of 5
Carbon has six protons. Which model shows a neutral atom of carbon?

Answers
Answer:
its d
Explanation:
Model D shows a neutral atom of carbon as it has 6 protons in the nucleus and 6 electrons in it's orbits.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
What is the electron configuration of the oxide ion
O
2
−
?
Answers
The electron configuration of an oxide ion O2− is represented by 1s2 2s2 2p6. The oxide ion is formed by the gain of two electrons by an oxygen atom that leads to the completion of the outermost shell of the oxygen atom, and hence it attains the stable electronic configuration of the nearest noble gas, i.e., neon.
The oxide ion is a stable species that is commonly found in many compounds. For example, the oxide ion forms many different salts such as potassium oxide (K2O) and sodium oxide (Na2O), which are commonly used as a source of oxygen in industrial applications. It is also an important component of many minerals and rocks, such as quartz (SiO2) and hematite (Fe2O3).In conclusion, the electron configuration of an oxide ion O2− is 1s2 2s2 2p6, which is attained after the gain of two electrons by an oxygen atom.
know more about oxygen atom.
https://brainly.com/question/7716347
#SPJ11
Diffusion and Organelle Retake Activity
(please finish in 5-9 minutes)
Question:
Fill in the blank
Water loves _____ and ____.
This means water will
move ______ the direction of the salt or sugar.
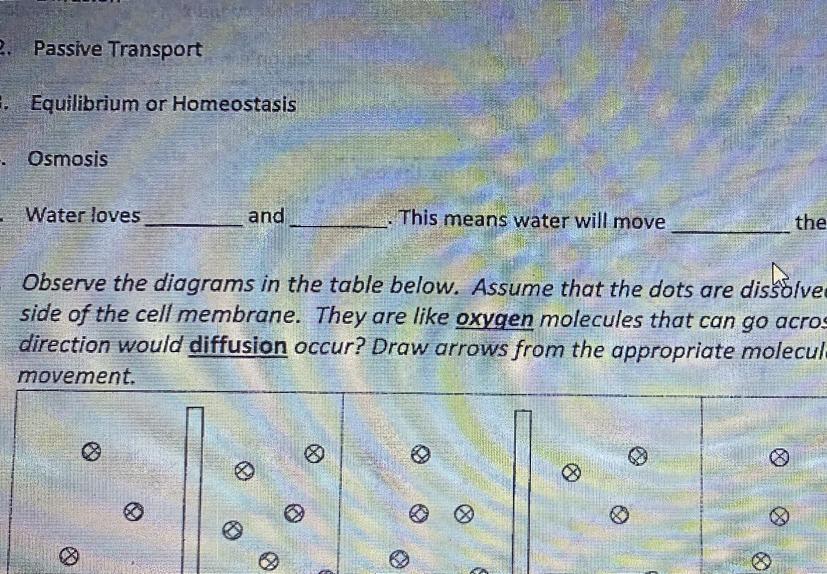
Answers
Answer:
wind and soil? north?
Explanation:
a 0.615 -g sample of unknown solid acid requires a total of 38.26 ml of 0.1032 m naoh to reach the endpoint. the mole ratio of unknown solid to naoh is 1:1 for the reaction. calculate the molar mass (g/mol) of the unknown solid.
Answers
The molar mass (g/mol) of the unknown solid is 155.36 g/mol.
A sample size of unknown solid acid, m = 0.615 g, The concentration of NaOH, c = 0.1032 m, The mole ratio of the unknown solid to NaOH is 1:1, The volume of NaOH used, V = 38.26 mL
We can calculate the number of moles of NaOH used as follows,
n = c * V / 1000 = 0.1032 * 38.26 / 1000 = 0.00395632 mol
Since the mole ratio of an unknown solid to NaOH is 1:1, we know that the number of moles of an unknown solid is also 0.00395632 mol.
The molar mass of the unknown solid can be calculated as follows,
M = m / n = 0.615 / 0.00395632 = 155.36 g/mol
Therefore, the molar mass of the unknown solid is 155.36 g/mol.
Know more about Molar mass here :
https://brainly.com/question/837939
#SPJ11
Hydrogen reacts with an element to form a compound. Which element would have the most valence electrons and also be able to react with hydrogen?
Answers
Answer:
chlorine
Explanation:
yup
Answer:
chlorine
Explanation:
Edge 2020
~theLocoCoco
The atoms of which elements tend to gain electrons? Which tend to lose electrons?
A.Metals tend to lose electrons; nonmetals tend to gain electrons.
B.Metals tend to gain electrons; nonmetals tend to lose electrons.
C.Most metals and nonmetals tend to lose electrons; metalloids tend to gain
electrons.
D.Metals and most nonmetals tend to lose electrons; noble gases tend to gain
electrons.
Answers
Answer:
Nonmetals tend to gain electrons and metals tend to lose electrons.
Explanation: