Mercury metal is poured into a graduated cylinder that holds exactly 20 mL. The mercury used to fill the cylinder has a mass of 306.0 g. From this information, calculate the density of mercury.
*
Don't forget the units!
Answers
Taking into account the definition of density, the density of mercury is 15.3 g/mL.
Definition of densityDensity is defined as the property that matter has to compress into a given space and it allows us to measure the amount of mass in a certain volume of a substance.
The expression for the calculation of density is:
density= mass÷ volume
It can be deduced that density is inversely proportional to volume: the smaller the volume occupied by a given mass, the higher the density.
Density of mercuryIn this case, you know that:
Mass= 306 gVolume= 20 mLReplacing in the definition of density:
density= 306 g÷ 20 mL
Solving:
density= 15.3 g/mL
In summary, the density of mercury is 15.3 g/mL.
Learn more about density:
brainly.com/question/952755
brainly.com/question/1462554
#SPJ1
Related Questions
select the single best answer. does q for the formation of 1 mol of no from its elements differ from q for the decomposition of 1 mol of no to its elements and what is the relationship between the two qs?
Answers
No, the Qs for the formation of 1 mol of no from its elements differs from q for the decomposition of 1 mol are the same.
What are decomposition and examples?
Decomposition reactions occur when complex chemical entities split apart into smaller components. Decomposition reactions often demand energy input. For instance, the thermal breakdown of potassium chlorate (KClO3) is a typical laboratory technique to generate oxygen gas.
How do substances decompose?
A chemical compound will separate into two or more products when its bonds are disrupted. When this occurs, a chemical event known as chemical breakdown takes place.
Learn more about decomposition here:
brainly.com/question/27300160
#SPJ4
What is the formula for dimensional analysis?
Answers
Answer:
Q=M,L,T then MLT is called the dimension formula and the components a,b,c are called the dimension
calculate the molar concentration (m), molality (m), and % by mass (% m), for a solution formed by mixing 10.7 g of a solute, with a molar mass of 86 g/mol, with 155.7 g of solvent. (the density of the solution is 1.3 g/ml).
Answers
To calculate the molar concentration (m), we need to determine the number of moles of the solute and the volume of the solution.
First, let's calculate the number of moles of the solute:
Moles of solute = Mass of solute / Molar mass of solute
= 10.7 g / 86 g/mol
= 0.1244 mol
Next, let's calculate the volume of the solution:
Volume of solution = Mass of solvent / Density of solution
= 155.7 g / 1.3 g/ml
= 119.77 ml
Now, we can calculate the molar concentration (m):
Molar concentration (m) = Moles of solute / Volume of solution (in liters)
= 0.1244 mol / (119.77 ml / 1000 ml/L)= 1.038 MTo calculate the molality (m), we need to determine the mass of the solvent and the mass of the solute.
Mass of solvent = 155.7 gMass of solute = 10.7 gMolality (m) = Moles of solute / Mass of solvent (in kg)
= 0.1244 mol / (155.7 g / 1000 g/kg)= 0.7988 mTo calculate the percent by mass (% m), we need to determine the mass of the solute and the mass of the solution.
Mass of solute = 10.7 g
Mass of solution = Mass of solute + Mass of solvent
= 10.7 g + 155.7 g= 166.4 gPercent by mass (% m) = (Mass of solute / Mass of solution) * 100
= (10.7 g / 166.4 g) * 100= 6.43%Therefore, the molar concentration (m) is 1.038 M, the molality (m) is 0.7988 m, and the percent by mass (% m) is 6.43%.
Learn More About Moles at brainly.com/question/29367909
#SPJ11
Which factor plays the biggest role in delaying the detection of childhood
diseases?
Answers
Answer:
poor access to health care providers
Explanation:
without health care providers you cant get tested.
How many atoms are in 10.4 mol of CI?
Answers
Answer:
there are two atoms
1. hydrogen
2.and water
Answer:
\(\boxed {\boxed {\sf 6.26 \times 10^{24} \ atoms }}\)
Explanation:
To convert from moles to atoms, we must use a number called Avogadro's Number which is:
\(6.022\times10^{23}\)
This number tells us the amount of atoms in a mole.
\(1 \ mole = 6.022\times10^{23} \ atoms\)
To convert from moles to atoms, we must multiply Avogadro's number by the amount of moles.
We know there are 10.4 moles of Chlorine. Multiply 10.4 (molar amount) by 6.022 * 10²³ (Avogadro's Number)
\(10.4 \ mol *\frac{6.022\times10^{23} \ atoms }{ 1 \ mole}\)
\(10.4 * 6.022\times10^{23} \ atoms\)
\(1 \ mole = 6.022\times10^{23} \ atoms\)
\(6.26288 \times 10^{24} \ atoms\)
Let's round to 3 significant figures, because the original measurement 10.4, has 3 sig figs (1, 0, and 4)
For the number 6.26288, 3 significant figures is the hundredths place. The 2 in the thousandth place tells us to keep the 6 in the hundredths place.
\(6.26 \times 10^{24} \ atoms\)
There are about 6.26 × 10²⁴ atoms in 10.4 moles of chlorine.
can the element oxygen form a molecule O² . interpret why so
Answers
Answer:
Yes, it can
Find the explanation below
Explanation:
A molecule is a substance made up of more than one atom. Most times, the individual atoms of elements are UNSTABLE, hence, their atoms get chemically bonded to one another to form a MOLECULE.
Oxygen is an element denoted by the symbol O. Oxygen is made up of atoms that becomes chemically joined together via covalent bonds i.e each atom of oxygen shares their valence electrons, to form OXYGEN MOLECULE (O2). Oxygen molecule is referred to as a diatomic molecule because it is made up of two of the same atoms.
how many atoms are in a body-centered cube, assuming that all atoms occupy lattice points?
Answers
The number of atoms in a body-centered cube, assuming that all atoms occupy lattice points, is two.
In a body-centered cube, there is one atom at each of the eight corners and one atom at the center of the cube. Since each atom only occupies one lattice point, the total number of atoms is simply the sum of the atoms at the corners and the center, which is equal to two.
In conclusion, a body-centered cube has a total of two atoms when all atoms occupy lattice points.
In a body-centered cube, each of the eight corners of the cube has one atom, and one additional atom is located at the center of the cube. This arrangement creates a lattice structure in which the atoms are evenly spaced apart from one another.
To understand how many atoms are in a body-centered cube, it's important to know how to calculate the total number of lattice points in the cube. The lattice points are the locations where the atoms are located in the cube. In a body-centered cube, there is one lattice point at each of the eight corners, and one lattice point at the center of the cube. This gives a total of nine lattice points in the cube.
However, not all lattice points are occupied by atoms. In a body-centered cube, each atom only occupies one lattice point. Therefore, the total number of atoms is simply the sum of the atoms at the corners and the center, which is equal to two.
In conclusion, a body-centered cube has a total of two atoms when all atoms occupy lattice points.
To know more about lattice points visit:
brainly.com/question/29774529
#SPJ11
if 100.0 mL of liquid weighs 81.23g what is the density of the liquid
Answers
Answer:
812.3 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
Consider an enzymatic reaction in which the initial concentration of substrate is low. If the amount of enzyme is held constant, but the amount of substrate is increased, the rate of an enzyme catalyzed reaction will.
Answers
Answer:69.8
Explanation:
please help quickly
Rubia was given a type of inclined plane called a ramp in her science class. Her teacher told her that the ramp should have a mechanical advantage of 3.
Rubia pulled a block up the ramp, but afterward she calculated that the mechanical advantage of the ramp was 2.8 instead of 3. Her teacher said she did not make a mistake. What did Rubia calculate?
A.
the actual operating power of the ramp
B.
the actual mechanical advantage of the ramp
C.
the ideal mechanical advantage of the ramp
D.
the ideal operating power of the ramp
Answers
Answer: B. The actual mechanical advantage of the ramp.
Explanation:
The professor saying that the ramp SHOULD have a mechanical advantage of 3 indicates that that would be the ideal mechanical advantage. Since Rubia calculated it to be 2.8, that means that it is the acutal mechanical advantage. The actual advantage will be reduced from the ieal due to things like friction.
A compass is placed near a certain type of metal. The needle on the compass moves. What type of force causes the needle to move SC. 6. P. 13. 1
Answers
A magnetic force is what moves the compass needle when it is in close proximity to a particular kind of metal. This is so because the magnetic fields of the metal item and the compass needle interact to create a force.
Permanent magnets, electric currents, and various types of metals may all be surrounded by magnetic fields, which are created by moving charges like electrons. The compass needle will move or align itself with the magnetic field lines when a magnetic field is applied to a magnetic substance, such as that material.If the compass is placed next to a metal item, the metal must likewise have a magnetic field or be able to create one when exposed to one. The compass needle moves as a result of the force created by the interaction of the magnetic fields, revealing the existence and direction of the magnetic field generated by the metal item.
learn more about compass needle here:
https://brainly.com/question/2577109
#SPJ4
How do you make potassium chlorate formula?
Answers
Potassium chlorate can be made by the process of adding chlorine, gas, hot sulfuric acid, and then filtering and drying the precipitate.
Potassium chlorate is a chemical compound that is commonly used in the production of matches, fireworks, and explosives. The formula for potassium chlorate is KClO3, which indicates that it is composed of one potassium ion (K+), one chlorine ion (Cl-), and three oxygen atoms.
To make potassium chlorate, you can start with a solution of potassium chloride (KCl) and add chlorine gas (Cl2) and hot concentrated sulfuric acid (H2SO4) to the solution. This will cause a chemical reaction to occur, which produces potassium chlorate as a precipitate.
The chemical reaction can be represented by the following equation:
6KCl + 6Cl2 + 6H2SO4 → 6KHSO4 + 3O2 + 2H2O
The potassium chlorate will appear as a white crystalline solid, which can be filtered and washed with water to remove any impurities. The resulting potassium chlorate can be dried and used for various applications. It is important to handle potassium chlorate with care, as it is a strong oxidizing agent and can be hazardous if mishandled.
Learn more about potassium chlorate here:
https://brainly.com/question/27429457
#SPJ4
How many electrons are in the calcium ion created by the calcium atom losing two electrons?
Answers
Calcium ion created by the calcium atom losing two electrons has 18 electrons.
A neutral calcium atom has 20 electrons. When it loses two electrons, it becomes a calcium ion with a +2 charge. Since electrons have a negative charge, a calcium ion with a +2 charge will have 2 fewer electrons than a neutral calcium atom.
The calcium ion is a positively charged ion that has lost two electrons from the neutral calcium atom. It has a 2+ charge and is represented as Ca2+. The electronic configuration of the calcium ion is 1s² 2s² 2p⁶ 3s² 3p⁶, which means it has a total of 18 electrons. Thus, a calcium ion with a +2 charge will have 18 electrons.
To know more about the calcium ion, here
brainly.com/question/12985536
#SPJ4
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
using the dumas method, it was determined that an unknown liquid had a molar mass of 72.01 g/mol. suppose that 3.573 grams of the unknown liquid vaporized in a 3.049 l flask at a particular temperature. if the pressure was measured to be 1.000 atm, what would you expect the temperature of the vapor to be (k)? type answer:
Answers
The expected temperature of the vapor is 569 K.
n = m/M
where n is the number of moles, m is the mass, and M is the molar mass.
n = 3.573 g / 72.01 g/mol = 0.0496 mol
Next, we can calculate the volume of the vapor using the volume of the flask:
V = 3.049 L
Now we can rearrange the ideal gas law to solve for the temperature:
T = PV/nR
Plugging in the values:
T = (1.000 atm) x (3.049 L) / (0.0496 mol x 0.0821 L·atm/mol·K)
T = 569 K
Temperature is a measure of the average kinetic energy of the particles in a substance. In chemistry, temperature plays a crucial role in determining the behavior of chemical reactions, physical changes, and phase transitions. As temperature increases, the kinetic energy of the particles in a substance also increases, leading to an increase in the rate of chemical reactions and a decrease in the viscosity of liquids.
The standard unit of temperature in chemistry is Kelvin (K), although Celsius (°C) and Fahrenheit (°F) are also used. The Kelvin scale is based on the absolute zero point, which is the theoretical temperature at which all molecular motion stops. In contrast, the Celsius and Fahrenheit scales are based on the freezing and boiling points of water at standard pressure, respectively.
To know more about Temperature refer to-
brainly.com/question/12085369
#SPJ4
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
why is it better to use deionized water in chemistry experiments
Answers
in the following atomic model, where does the strong nuclear force happen? outside a between a and b between b and c inside c
Answers
In the given atomic model, the strong nuclear force occurs inside the nucleus. Option D is correct.
The strong nuclear force is one of the fundamental forces of nature and it acts on particles called quarks, which are the building blocks of protons and neutrons. This force is responsible for holding the nucleus of an atom together.
Inside the nucleus, protons and neutrons are tightly bound to each other by the strong nuclear force. It overcomes the electrostatic repulsion between the positively charged protons, preventing the nucleus from disintegrating. The strong nuclear force is extremely powerful but short-ranged, meaning it acts only at very short distances within the nucleus.
Outside the nucleus, in the electron cloud, other forces such as electromagnetic forces and gravitational forces dominate the interactions. However, within the nucleus, the strong nuclear force is responsible for binding the protons and neutrons together, maintaining the stability of the atomic nucleus.
Hence, D. is the correct option.
To know more about atomic model here
https://brainly.com/question/1100068
#SPJ4
--The given question is incomplete, the complete question is
"In the following atomic model, where does the strong nuclear force happen? A) outside a B) between a and b C) between b and c D) inside c."--

Ruth rode home at a constant speed for the first 10 minutes of the trip. What was her constant speed?
______ m/min
What was Ruth's average speed for the entire trip?
______ m/min
Ruth stopped to talk with another friend during the trip.
How far was she from home when she stopped?
______ m
How long was she stopped?
_____ min
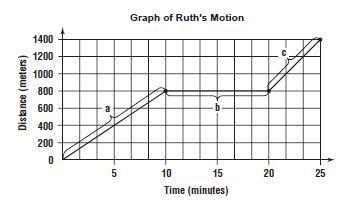
Answers
From the graph, the distance for the first 10 minutes is 600 meters.
10 minutes = 10 x 60 = 600 seconds
Ruth's speed for the first 10 minutes = distance/time
= 600/600 = 1 m/s
Average speed = total distance traveled/total time taken
Total distance = 1400 meters
Total time = 25 minutes = 25 x 60 = 1500 seconds
Average speed = 1400/1500 = 0.93 m/s
Ruth had traveled 800 meters before stopping to talk to her friend:
1400 - 800 = 600 meters.
Thus, she was 600 meters away from home when she stopped.
She stopped between 10 and 20 minutes:
20 - 10 = 10 minutes
Thus, she stopped to talk to her friend for 10 minutes.
More on velocity-time graphs can be found here: https://brainly.com/question/11290682
#SPJ1
Water, H2O, is a molecule made of oxygen and hydrogen. The bonds that hold water molecules together are due to shared ___________, and known as covalent bonds. Question 12 options: electrons neutrons photons protons.
Answers
Oxygen and hydrogen share electrons in the molecule of water to form covalent bonds.
What kinds of bonds exist?Covalent bonds: These are formed between nonmetals and electrons by sharing electrons.Ionic bonds: These are formed between metals, which lose electrons, and nonmetals, which gain electrons.Metallic bonds: There are formed between metals. Electrons are delocalized in a cloud.Water, H₂O, is a molecule made of 2 nonmetals: oxygen and hydrogen. The bonds that hold water molecules together are due to shared electrons, and known as covalent bonds.
Oxygen and hydrogen share electrons in the molecule of water to form covalent bonds.
Learn more about chemical bonds here: https://brainly.com/question/6071754
Where can mermaids be found in Ghana and how can you be a mermaid if you want to?
Answers
There is no explicit proof mermaids exist, but animals and possibly even objects have been mistaken for them. An instance of this would be the claim someone had that they spotted a real mermaid in a beach of South Africa and also in Northern Ireland. Mermaids can be mistaken for a lot of animals, the most notable being a sea cow.
There are claims that mermaids (which also were given the moniker “maame water) have been spotted in a lot of places, but as far as evidence goes, we don’t know if this is real or not. Until then, we shall continue to say it isn’t; just like in court where before a person is proven guilty, they’re innocent!
Convert 0.05 Mm -> mm
Answers
Answer:
To convert any value in millimeters to millimeters, just multiply the value in millimeters by the conversion factor 1. So, 0.05 millimeter times 1 is equal to 0.05 millimeters.
Answer:
0.00005
Explanation:
Which quantity of particles is correctly represented 1 mol of H2?
Answers
quantity of caco3 required to make 100 ml of a 100 ppm ca2 solution
Answers
To determine the quantity of CaCO3 required to make 100 mL of a 100 ppm Ca2+ solution, 2.777 mg of CaCO3 is required.
First, calculate the amount of Ca2+ ions required in 100 mL of solution:
(100 mL / 1000 mL) x 100 mg = 10 mg of Ca2+ ions
Next, determine the mass ratio of Ca2+ ions to CaCO3. The molecular weight of Ca2+ is 40.08 g/mol and that of CaCO3 is 100.09 g/mol. Therefore, the mass ratio is 40.08/100.09.
Finally, calculate the amount of CaCO3 required to obtain 10 mg of Ca2+ ions:
(10 mg Ca2+ ions) x (100.09 g CaCO3 / 40.08 g Ca2+) ≈ 2.777 mg of CaCO3
So, 2.777 mg of CaCO3 is required to make 100 mL of a 100 ppm Ca2+ solution.
To learn more about mass ratio visit:
brainly.com/question/14577772
#SPJ11
what is the predominant sweetener used in formulating beverages?
Answers
The predominant sweetener used in formulating beverages is high-fructose corn syrup (HFCS).
High-fructose corn syrup (HFCS) is a common sweetener widely used in the formulation of beverages. It is derived from corn starch and consists of varying proportions of glucose and fructose. The most common types of HFCS used in beverages are HFCS-55 (which contains approximately 55% fructose and 45% glucose) and HFCS-42 (which contains approximately 42% fructose and 58% glucose).
HFCS is favored in the beverage industry due to its sweetness, affordability, and ease of use as a liquid sweetener. It dissolves easily in water and can be added to a wide range of beverage formulations, including carbonated drinks, fruit juices, sports drinks, and flavored waters.
While HFCS is a prevalent sweetener, it is important to note that other sweeteners such as sucrose (table sugar), artificial sweeteners (e.g., aspartame, sucralose), and natural sweeteners (e.g., stevia, monk fruit extract) are also used in beverage formulations, depending on factors such as taste preferences, cost, and desired product characteristics.
learn more about high-fructose corn syrup here:
https://brainly.com/question/32821004
#SPJ11
Reversible protein phosphorylation controls the activity, structure, and cellular localization of many types of proteins. What class of enzymes are responsible for adding phosphoryl groups to proteins? What class of enzymes removes them?
Which amino acids are able to be phosphorylated?
List three general ways in which the covalent addition of a phosphate group to an amino acid side chain can effect conformational change of a protein.
Answers
Reversible protein phosphorylation controls the activity, structure, and cellular localization of many types of proteins. Protein kinases add a phosphoryl group to proteins while protein phosphatases remove them.
The amino acids that are able to be phosphorylated are the hydroxyl-containing side chains of serine, threonine, and tyrosine. The covalent addition of a phosphate group to an amino acid side chain can effect conformational change of a protein in three general ways:
1. Charge change: The negatively charged phosphate group will change the electrostatic properties of the amino acid side chain to which it is attached, thus disrupting salt bridges, and charge interactions that stabilize the protein's native conformation.
2. Steric hindrance: The addition of a phosphate group increases the size of the amino acid side chain, which can create steric hindrance. This can introduce a kink or bend in the polypeptide chain that leads to conformational changes of the protein.
3. Hydrogen bonding: The addition of a phosphate group to an amino acid side chain can introduce a hydrogen-bonding group into the protein structure.
To know more about Protein kinases, refer
https://brainly.com/question/7983530
#SPJ11
I know that C is a correct answer. But is F correct as well?

Answers
Answer: Yes F is also correct.
Explanation:
Which of the two processes shown in the following equations requires shorter-wavelength radiation?
(a) Na+(g) → Na2+(g) + e−
(b) Na(g) → Na+(g) + e−
Answers
Answer: The process that requires shorter-wavelength radiation is process (b):
(b) Na(g) → Na+(g) + e−
Explanation:
In this process, a neutral sodium atom (Na) loses an electron to form a sodium ion (Na+). The transition involves the removal of an electron from the atom, resulting in an increase in the ionization energy. To accomplish this, shorter-wavelength radiation with higher energy is required.
In contrast, process (a) involves the removal of an additional electron from a sodium ion (Na+), forming a doubly ionized sodium ion (Na2+). Since the ionization energy typically increases with the successive removal of electrons, the process (a) would require radiation with longer wavelengths and lower energy compared to process (b).
To learn more about Ionization Energy from the given link
https://brainly.com/question/20658080
#SPJ4
The process that requires shorter-wavelength radiation is process (b): Na(g) → Na+(g) + e−
In this process, a neutral sodium atom (Na) loses an electron to form a sodium ion (Na+). The transition involves the removal of an electron from the atom, resulting in an increase in the ionization energy. To accomplish this, shorter-wavelength radiation with higher energy is required.
In contrast, process (a) involves the removal of an additional electron from a sodium ion (Na+), forming a doubly ionized sodium ion (Na2+). Since the ionization energy typically increases with the successive removal of electrons, the process (a) would require radiation with longer wavelengths and lower energy compared to process (b).
To learn more about Ionization Energy from the given link
brainly.com/question/20658080
#SPJ4
A recipe calls for 3 tablespoons of milk for 7 pancakes. If this recipe was used to make 28 pancakes, how many tablespoons of milk would be needed
A. 15
B. 11
C. 12
D. 9
Answers
The number of tablespoons of milk needed for 28 pancakes is determined as 12 tablespoons.
option C is the correct answer.
How many tablespoons of milk would be needed?The number of the tablespoons of milk that would be needed is calculated by applying simple proportion method.
3 tablespoons of milk for 7 pancakes;
3 -----------> 7
? tablespoons of milk for 28 pancakes;
? --------------------> 28
Combine the two equations and solve for the number of tablespoons needed as follows;
? = ( 3 x 28 ) / 7
? = 12
Thus, The number of tablespoons of milk needed for 28 pancakes is determined by applying simple proportion.
Learn more about proportion here: https://brainly.com/question/19994681
#SPJ1
Molecules at a higher temperature have _______ kinetic energy, speed, and momentum compared to molecules at a lower temperature.
Answers
Answer:
Molecules at a higher temperature have higher/more kinetic energy, speed, and momentum compared to molecules at a lower temperature.
Explanation: