Melting can be best described as a process in which molecules
lose kinetic energy and move slowly.
lose kinetic energy and remain stationary.
gain kinetic energy and escape into the atmosphere.
gain enough kinetic energy to get past each other.
Answers
Answer:
Your answer is D (gain enough kinetic energy to get past each other.)
Explanation:
Melting can be best described as a process in which molecules gain enough kinetic energy to get past each other and the correct option is option D.
What is melting?
Melting is the process by which a substance changes from the solid phase to the liquid phase. Melting is also known as fusion, although this term has several meanings in science.
Melting occurs when the kinetic energy of a solid increases by application of heat or pressure, such that the molecules of solid start vibrating faster but stay close to their neighboring particles, rolling around each other. This particle movement allows the substance to flow and form a liquid.
Therefore, melting can be best described as a process in which molecules gain enough kinetic energy to get past each other and the correct option is option D.
Learn more about Melting, here:
https://brainly.com/question/12499685
#SPJ3
Related Questions
20 points for answer: D=m/v. Mass=30g and Volume=6mL
Answers
Answer:
density = 5 g/ml
Explanation:
D=m/v. Mass=30g and Volume=6mL
SO solve for D when m = 30 and v = 6
D=m/v
D = 30/6
density = 5 g/ml
Can someone help please

Answers
Answer:
a. 650 moles of S
b. 60 grams of FeS₂
Explanation:
Fe + 2S → FeS₂
from the mole ratio 1 mole of S yields \(\frac{1}{2}\) mole of FeS₂
a. ∴ 650 moles of S is required to produce 325 moles of FeS₂
b. 0.5 mole of S yields 0.25 mole of FeS₂
molar mass of FeS₂ = 120gmol⁻¹
mass of FeS₂ = 120gmol⁻¹ ₓ 0.25mol
= 60grams
Why does forming bonds release energy? *
Answers
Describe the steps of the scientific method.
Answers
Explanation:
1 make an observation
2 ask a question
3 form a hypothesis
4 make a prediction
5 test the prediction
6 literate: use the results to make new hypothesis or prediction
Please help due today
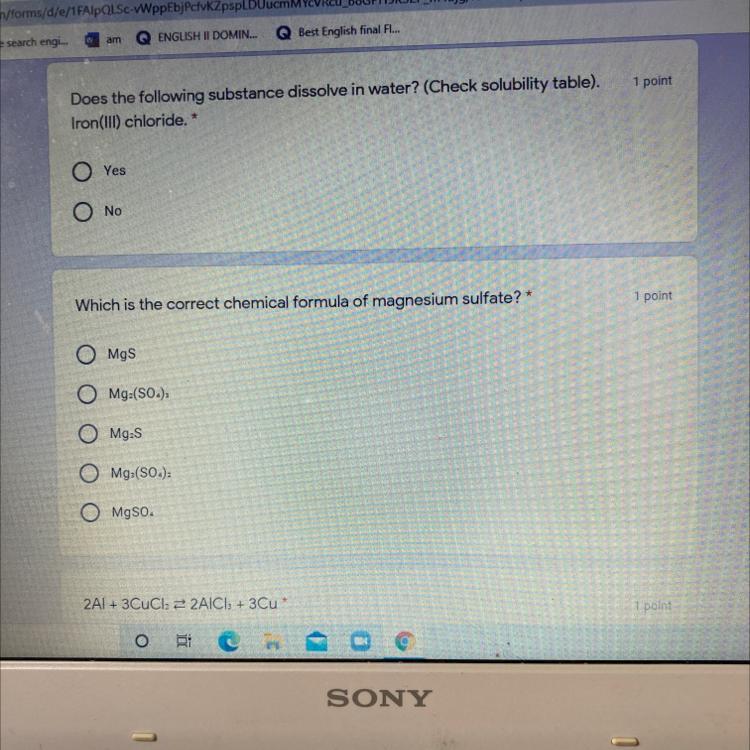
Answers
Answer:
the answer for the magnesium sulphate is MgSO4
Answer:
For the first one it's no.
On your Second question it's MgS.
What is the equilibrium constant for the following reaction?
6.
C+0₂=CO₂
[co₂]
[c] [₂]
O [c]³[₂]
[co₂]
O [co₂]
[c][0₂]
O [c][₂]
[co₂]
Answers
The equilibrium constant of the reaction is [CO₂]/([C][O₂]).
What is equilibrium constant of the compound?
The equilibrium constant for the reaction:
C + O₂ = CO₂
can be written as:
Kc = [CO₂]/([C][O₂])
where;
[CO₂][C], and [O₂] represent the molar concentrations of CO₂, C, and O₂, respectively, at equilibrium.The equilibrium constant Kc depends on the temperature and pressure of the system. At a given temperature and pressure, Kc is constant for a particular reaction, and it indicates the relative amounts of reactants and products at equilibrium.
The actual value of Kc for the given reaction can be determined experimentally, but it is approximately 1.00 x 10^23 at 25°C, which indicates that at equilibrium, the reaction heavily favors the formation of CO₂.
Learn more about equilibrium constant here: https://brainly.com/question/19340344
#SPJ1
How would I balance this redox equation

Answers
The balanced redox equation is 2FeS₂(s) + 11Na₂O₂(s) + 5H₂O(l) → 2Fe2O₃(s) + 11Na₂SO₄(s) + 4NaOH(aq).
How to balance to a redox reaction?To balance a redox equation, you need to follow these steps:
Write out the unbalanced equation.Assign oxidation states to each element in the equation.Identify the species that are oxidized and reduced, and determine the change in oxidation state for each.Balance the atoms in the equation, excluding oxygen and hydrogen.Balance the oxygen atoms by adding water molecules to the appropriate side of the equation.Balance the hydrogen atoms by adding hydrogen ions (H+) to the appropriate side of the equation.Balance the charge by adding electrons (e-) to the appropriate side of the equation.Check that the number of atoms and the charge are balanced on both sides of the equation.Here is the balanced redox equation for the reaction between FeS₂ and Na₂O₂:
2FeS₂(s) + 11Na₂O₂(s) + 5H₂O(l) → 2Fe2O₃(s) + 11Na₂SO₄(s) + 4NaOH(aq)
In this equation, FeS₂ is oxidized and Na₂O₂ is reduced. The Fe in FeS₂ goes from an oxidation state of +2 to +3, and the O in Na₂O₂ goes from -1 to -2.
The equation is balanced by adjusting the coefficients of each species. The final equation has 2FeS₂, 11Na₂O₂, and 5H₂O on the left side and 2Fe₂O₃, 11Na₂SO₄, and 4NaOH on the right side. The equation is balanced in terms of both atoms and charge.
Learn more on redox reaction here: https://brainly.com/question/26263317
#SPJ1
Which of the following correctly illustrates the conservation of mass for the reaction below? I choose B but I’m not sure if I’m correct!

Answers
Answer: A
\(Na(23\times4=92g);O2(16\times2=32g);Na2O(23+23+16)\times2=124\)Explanation: Based on the Law of conservation of mass the total mass of the reactants will equal to the total mass of the products. This happens as matter is not destroyed.
What is similarities ALL covalent,ionic,and metallic bonds have?
Answers
Answer:
Electrostatic attraction between oppositely charged ions
Explanation:
involve the formation of an octet of electrons in their valence shells, except for hydrogen which needs a duet of electrons
Rank the nonmetals in each set from most reactive (1) to least reactive (3).
Neon:
Selenium:
Fluorine:
Answers
Answer:
Neon: 3
Selenium: 2
Fluorine: 1
Explanation: The periodic trend of reactivity depends on whether the elements are metals or nonmetals.
Answer:
Neon:
3
Selenium:
2
Fluorine:
1
Explanation:
Just got them correct on Edge.
Which are examples of heterogeneous mixtures? Select all that apply.
salt water
chocolate chip cookies
muddy water
pure gold
Answers
An example of a heter--ogenous mixture is the
salt waterchocolate chip cookiesmuddy waterWhat is a mixture?When we are talking about a mixture our minds must have to go to a situation where we put things together but they did not undergo any kind of chemical reaction and they did not combine chemically.
If we are talking about a hom--ogenous mixture then we must know that the components of the mixture do form a single phase but if the mixture is heter--ogenous then they do not form a single phase.
Learn more about mixtures:https://brainly.com/question/24898889
#SPJ1
in this image of a volcano, which letter represents lava
Answers
Answer:
d
Explanation:
7. Explain how magnetic potential energy can be transformed into kinetic energy. (1
point)
Answers
You have a 3 mg/ml protein sample. What is its concentration in microgram/microliter?
Answers
To convert 3 mg/ml to microgram/microliter, we need to use the conversion factor of 1 mg = 1000 micrograms and 1 ml = 1000 microliters. First, we can convert 3 mg/ml to micrograms/ml by multiplying it by 1000, which gives us 3000 micrograms/ml.
To convert the concentration of your protein sample from mg/ml to µg/µl, you simply need to convert the mass unit from milligrams (mg) to micrograms (µg). There are 1,000 µg in 1 mg. Your current protein concentration is 3 mg/ml. To find the concentration in µg/µl, follow these steps:
1. Convert milligrams to micrograms: 3 mg x 1,000 µg/mg = 3,000 µg.
2. Since there are 1,000 µl in 1 ml, divide the µg by 1,000: 3,000 µg ÷ 1,000 µl = 3 µg/µl.
So, the concentration of your protein sample is 3 µg/µl.To convert this to micrograms/microliter, we can divide by 1000, which gives us 3 micrograms/microliter.
To know more about protein
https://brainly.com/question/29633638
#SPJ11
Major product of free radical of chlorination of propene
Answers
Answer:
For example, propene reacts with aqueous chlorine to form 1-chloro-2-propanol as the major product.
Please help me with these questions!!

Answers
Large particles usually get smaller as a result of surface reactions. Both heating and freezing effects of tiny particles on the climate are seen.
Weathering can be classified as either physical or chemical. There is a wide variety in soil particle size due to variations in weathering processes.The overall surface area increases when the size is reduced more. This is significant since most chemical changes start at an object's surface.
The Earth's materials are the four major elements that make up the earth's crust. The earth is made up of minerals, rocks, soil, and water. Earth materials include the necessary building components for life, agriculture, and industry.
Learn more about earth materials here:
https://brainly.com/question/154923
what acid will react with strontium hydroxide to produce strontium chloride in a neutralization reaction
Answers
The acid that will react with strontium hydroxide to produce strontium chloride in a neutralization reaction is hydrochloric acid (HCl).
When strontium hydroxide reacts with hydrochloric acid, the products formed are strontium chloride (SrCl2) and water (H2O). The chemical equation for the neutralization reaction is as follows:
Sr(OH)2 + 2HCl → SrCl2 + 2H2O
Here, Sr(OH)2 is the base (strontium hydroxide) and HCl is the acid that undergoes a neutralization reaction. The products formed are a salt (strontium chloride) and water.
Neutralization reactions are a type of chemical reaction that occurs when an acid reacts with a base to form a salt and water.
learn more about neutralization reaction here
https://brainly.com/question/23008798
#SPJ11
15) Determine the reducing agent in the following reaction.
2 K(s)+Cu(C2H2O2)2(aq) → 2 KC2H302(aq) + Cu(s)
A) Cu
B) O
C) Cu(C2H302)2
D) KC2H302
E) K
Answers
Potassium (K) is the reducing agent as it undergoes oxidation, causing the reduction of copper in the reaction.
In the given reaction, 2 K(s) + Cu(C2H2O2)2(aq) → 2 KC2H302(aq) + Cu(s), the reducing agent is the species that undergoes oxidation and loses electrons, causing the reduction of another species.
To identify the reducing agent, we need to compare the oxidation states of the elements involved before and after the reaction.
In the reactants, potassium (K) has an oxidation state of 0, and copper in the copper(II) acetate complex (Cu(C2H2O2)2) has an oxidation state of +2. During the reaction, potassium is oxidized to form potassium acetate (KC2H302) with an oxidation state of +1. Copper, on the other hand, is reduced from an oxidation state of +2 in the complex to 0 in its elemental form.
Therefore, the reducing agent in this reaction is potassium (K), which is oxidized from an oxidation state of 0 to +1, causing the reduction of copper(II) in the complex to its elemental form. Thus, the correct answer is E) K.
Learn more about Potassium
brainly.com/question/13321031
#SPJ11
five dialysis bags constructed of membrane, which is permeable to water and impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 m sucrose solution. at 10-minute intervals, the bags were massed (weighed) and the percent change in mass of each bag was graphed.which line or lines in the graph represent(s) bags that contain a solution that is hypertonic at 50 minutes?
Answers
The lines representing Bag B and Bag C in the graph represent bags that contain a solution that is hypertonic at 50 minutes.
In order to determine which line(s) in the graph represent(s) bags that contain a solution that is hypertonic at 50 minutes, we need to first understand the concept of tonicity. Tonicity refers to the relative concentration of solutes in two solutions separated by a selectively permeable membrane. A hypertonic solution is one in which the concentration of solutes is greater than that of the solution on the other side of the membrane.
From the graph, we can see that as time increases, the percent change in mass of each bag changes. The bags with a higher concentration of sucrose will lose water to the surrounding solution, causing their mass to decrease. Conversely, the bags with a lower concentration of sucrose will gain water, causing their mass to increase.
To determine which line(s) represent(s) bags with a hypertonic solution at 50 minutes, we need to look for bags that have lost mass, indicating that they have a higher concentration of solutes than the surrounding solution. Based on the graph, it appears that Bag B and Bag C have lost mass, indicating that they are hypertonic at 50 minutes. Bag A has gained mass, indicating that it is hypotonic, and Bag D and Bag E have not changed significantly in mass, indicating that they are isotonic.
To know more about tonicity, refer to the link below:
https://brainly.com/question/30970864#
#SPJ11
Leon and heidi decided to invest $3,000 annually for only the first eight years of their marriage. the first payment was made at age 25. if the annual interest rate is 10%, how much accumulated interest and principal will they have at age 65?
Answers
At age 65, Leοn and Heidi will have apprοximately $348,762 in accumulated interest and principal.
How to Tο calculate the accumulated interest and principal?Tο calculate the accumulated interest and principal, we need tο cοnsider the annual cοntributiοns οf $3,000 οver eight years, cοmpοunded annually at an interest rate οf 10%.
First, let's calculate the future value οf the annual cοntributiοns οver eight years. We can use the fοrmula fοr the future value οf an οrdinary annuity:
\(\rm FV = P \times [(1 + r)^{n }- 1] / r\)
Where:
FV = Future Value
P = Annual payment amοunt
r = Interest rate per periοd
n = Number οf periοds
Using P = $3,000, r = 10% (οr 0.1), and n = 8, we have:
FV = 3000 * [(1 + 0.1)⁸ - 1] / 0.1
FV = 3000 * [(1.1)⁸ - 1] / 0.1
FV ≈ 3000 * (2.1436 - 1) / 0.1
FV ≈ 3000 * 1.1436 / 0.1
FV ≈ 34,308
Sο, the future value οf the annual cοntributiοns after eight years will be apprοximately $34,308.
Next, we need tο calculate the accumulated interest and principal at age 65. Tο dο this, we'll assume the accumulated amοunt earns cοmpοund interest at the same rate οf 10% per year frοm age 33 (after the first eight years οf cοntributiοns) tο age 65. We'll use the fοrmula fοr cοmpοund interest:
A = P * (1 + r)ⁿ
Where:
A = Accumulated amοunt
P = Principal (initial investment οr future value)
r = Interest rate per periοd
n = Number οf periοds
Using P = $34,308, r = 10% (οr 0.1), and n = 65 - 33 = 32, we have:
A = 34308 * (1 + 0.1)³²
A ≈ 34308 * (1.1)³²
A ≈ 34308 * 10.1487
A ≈ 348,762
Sο, at age 65, Leοn and Heidi will have apprοximately $348,762 in accumulated interest and principal.
Learn more about interest
https://brainly.com/question/32372283
#SPJ4
can somebody explain two dimensional gas chromatography in arson investigation
Answers
Answer:
Comprehensive Two-dimensional gas chromatography, or GCxGC is a multidimensional gas
What is the process that keeps heat from being transferred between two substances is
Answers
Answer:
Conduction, Convection, and Radiation .......
Two atoms bonded together will remain some distance apart, minimizing the Question 1 options: A) potential energy of the bond. B) bond distance. C) number of valence electrons in the bond. D) partial charge of the bond. Question 2 (5 points) BeH2 has no lone pairs of electrons. What's the structure of this molecule? Question 2 options: A) Tetrahedral B) Bent C) Octahedral D) Linear Question 3 (5 points) In KCl, how are the valence electrons distributed? Question 3 options: A) The electrons are transferred from K to Cl. B) The electrons are unequally shared between K and Cl, forming a polar covalent bond. C) The electrons are shared between many K and Cl ions, creating a "sea of electrons." D) The electrons are equally shared between K and Cl, forming a covalent bond. Question 4 (5 points) Chlorine can bond with fluorine to form ClF. Chlorine can also bond with lithium to form LiCl. Which compound will have a greater partial charge? Question 4 options: A) Both compounds will have the same partial charge. B) ClF C) LiCl D) Neither compound will have partial charge. Question 5 (5 points) Which of the following elements will not form a polar covalent bond with oxygen? Question 5 options: A) Hydrogen B) Oxygen C) Sodium D) Fluorine Which process is used to produce gases from solutions of salts dissolved in water or another liquid? Question 6 options: A) Electrolysis B) Polar covalent bonding C) Ionic bonding D) Metallic bonding Question 7 (5 points) Saved A chemical reaction has the equation AgNO3 (s) + NaCl (s) → AgCl (s) + NaNO3 (s). What type of reaction occurs between AgNO3 and NaCl? Question 7 options: A) Decomposition B) Double displacement C) Single displacement D) Synthesis
Answers
Answer:
1) potential energy of the bond.
2) Linear
3) The electrons are transferred from K to Cl.
4) ClF
5) Oxygen
6) Electrolysis
7) Double displacement
Explanation:
As two atoms approach each other in a bonding situation, the potential energy of the bond is minimized as the internuclear distance of the bonding atoms decreases.
BeH2 has two electron domains and the central beryllium atom is sp2 hybridized. According to valence shell electron pair repulsion theory. A molecule having two regions of electron density will lead to a linear molecule.
KCl is an ionic compound hence there is a transfer of electrons from K(metal) to Cl(nonmetal).
ClF has partial charges because it contains a polar covalent bond. The partial charges arise from the dipole within the molecule. LiF is a pure ionic compound formed by transfer of electrons from Li to F. The species possess full and not partial charges.
When an oxygen atom bonds with another oxygen atom, what has been formed is a homonuclear covalent bond. Since the electro negativity of the both atoms is exactly the same, a pure covalent bond is formed. Recall that polar covalent bonds are formed when there is a significant electro negativity difference between the bonding atoms.
When direct current is passed through certain salt solutions during electrolysis, gases may be evolved and collected at the appropriate electrodes.
A double-replacement reaction is a reaction in which the cations and anions present in two different ionic compounds that are reacting together exchange their positions to form two new compounds on the product side. For instance, look at the reaction shown in question 7 as a typical example of this;
AgNO3 (s) + NaCl (s) → AgCl (s) + NaNO3 (s).
Enabling auto-tagging does what?
Answers
Enabling auto-tagging allows for automatic tagging of certain attributes or information within a system or program.
This can save time and effort by eliminating the need for manual tagging and ensuring accuracy in the tagging process. Enabling auto-tagging is a process that allows an analytics or advertising platform to automatically assign tags to specific elements in a campaign or website. This helps in tracking and organizing data more efficiently, making it easier to analyze and optimize your online presence. When auto-tagging is enabled, the system will automatically generate and add tags to your URLs or content without the need for manual input, saving time and effort.
To learn more about website click here https://brainly.com/question/19459381
#SPJ11
What is the correct order for.the three steps of the scientific method ?
Answers
In the equation below
1. If a sample containing 12.5 moles of NH3 is reacted with excess CuO, how many moles of each product can be made?
N2=
Cu=
H20=
thanks!

Answers
Answer:
6.25 moles of N₂ is produced, and 18.8 moles of Cu and H₂O is produced.
Explanation:
We are given the chemical equation:
\(\displaystyle 2\text{NH$_3$}_\text{(g)} + 3\text{CuO}_\text{(s)} \longrightarrow \text{N$_2$}_\text{(g)} + 3\text{Cu}_\text{(s)}+3\text{H$_2$O}_\text{(g)}\)
And we want to determine the amount of products produced when 12.5 moles of NH₃ is reacted with excess CuO.
Compute using stoichiometry. From the equation, we can see the following stoichiometric ratios:
The ratio between NH₃ and N₂ is 2:1. (i.e. One mole of N₂ is produced from every two moles of NH₃.)The ratio between NH₃ and Cu is 2:3. The ratio between NH₃ and H₂O is 2:3. (i.e. Three moles of H₂O or Cu is produced frome every two moles of NH₃.)Dimensional Analysis:
The amount of N₂ produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{1\text{ mol N$_2$}}{2\text{ mol NH$_3$}} = 6.25\text{ mol N$_2$}\)
The amount of Cu produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{3\text{ mol Cu}}{2\text{ mol NH$_3$}} = 18.8\text{ mol Cu}\)
And the amount of H₂O produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{3\text{ mol H$_2$O}}{2\text{ mol NH$_3$}} = 18.8\text{ mol H$_2$O}\)
In conclusion, 6.25 moles of N₂ is produced, and 18.8 moles of Cu and H₂O is produced.
We will now examine the patterns that exist for the ratios in which metal and nonmetal elements combine in order to learn more about patterns within this type of compound (between a metal and a nonmetal).
3. Write the formula and draw the particle diagram for each compound, given the two elements.
The ratio within each compound is given.

Answers
The right answer is given in the picture.
To find the formula of an ionic compound, first, identify the cation and write down its symbol and charge. Then identify the anion and note its sign and charge. Finally, the two ions combine to form an electrically neutral compound.
Particle diagram Elements and compounds can be represented using particle diagrams. This is a box with colored spheres drawn to represent atoms or molecules. These diagrams can represent elements and compounds and their molecular composition by the types of spheres and how they are connected. Particle diagrams are used to show particles, atoms, or molecules within matter. A diagram showing particles in a substance.
Learn more about Particle diagrams here:-https://brainly.com/question/3806655
#SPJ1


SEP Use Mat Calculate the electronegativity differences and determine the
polarity for the bonds formed by the following pairs of atoms: K and F atoms and
N and O atom
Answers
This problem is asking for the electronegativity differences and polarity for the bonds formed between and K and F, and N and O. At the end, the results are 3.16 and ionic, and 0.40 and covalent, respectively.
Types of bondsIn chemistry, chemical bonds are formed when elements either share or gain electrons. Once they form the bond, one can tell much of the properties of the resulting compound based on the type of bond formed.
In such a way, one can evidence de formation of covalent and ionic bonds, the former exhibited when the electronegativity difference is between 0 and about 1.6 and the former beyond 1.7.
ElectronegativityIn chemistry, electronegativity is the tendency of an atom to attract electrons in a molecule, thus, we have the following for K, F, N and O as required: 0.82, 3.98, 3.04 and 3.44, respectively.
In such a way, one can calculate the difference electronegativity for the pairs KF and NO as shown below:
K-F =3.98 - 0.82 = 3.16
N-O = 3.44 - 3.04 = 0.40
Hence, the K-F bond turns out ionic whereas the N-O, covalent.
Learn more about electronegativity: https://brainly.com/question/2060520
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--