List some important notes about fractional distillation...
Answers
Here are some important notes about fractional distillation:
1. Fractional distillation is a process used to separate a mixture of liquids based on their boiling points.
2. The process involves heating the mixture to vaporize it, and then condensing the vapors back into liquid form.
3. As the temperature of the mixture increases, the liquids with lower boiling points will vaporize first, and can be collected separately.
4. A fractionating column is used in the process to separate the different components of the mixture by their boiling points.
5. The fractionating column is packed with materials such as glass beads, which provide a large surface area for the vapors to condense and re-vaporize.
6. As the vapors rise through the fractionating column, they are repeatedly condensed and re-vaporized, with the components with higher boiling points condensing and falling back down the column, while the components with lower boiling points continue to rise and be collected separately.
7. The temperature of the mixture is carefully controlled throughout the process to ensure that the components are separated efficiently.
8. Fractional distillation is commonly used in the petroleum industry to separate crude oil into its various components, such as gasoline, diesel fuel, and kerosene.
9. It is also used in the production of alcoholic beverages, such as whiskey and rum, to separate and concentrate the alcohol content.
To know more about fractional distillation, please click on:
https://brainly.com/question/31829945
#SPJ11
Related Questions
Determine
the stoichiometry of the chemical equation written
below:(3pt)
1C3H8 + 5O2 → 3CO2 + 4H2O
Answers
Stoichiometrically, the mole ratio of C3H8 to O2 to CO2 to H2O is 1:5:3:4.
According to the equation, the mole ratio of C3H8 to O2 is 1:5. This means that 1 mole of C3H8 will require 5 moles of O2 for complete reaction.
Also, 1 mole of C3H8 when completely burned in 5 moles O2, will produce 3 moles of CO2 and 4 moles of H2O.
More on stoichiometry can be found here: https://brainly.com/question/9743981?referrer=searchResults
100 PTS IM DESPERATE NEED BY TOMMOROW


Answers
Answer:
rest will do later mom is calling me plz understand promise will do it
Explanation:
Elements and compounds are similar in that they are both made of atoms and in some cases molecules.Methods of Breaking Down CompoundsThe only way to break down a compound is through a chemical change. Sometimes, energy is needed for a chemical change to happen. Two ways to add energy to break down a compound are to apply heat and to apply an electric current.Answer:
lol
Explanation:
its 5 points
What do you observe at the bottom of the test tube after heating?
Answers
Answer:
If you heat the test tube from the bottom, any liquid can turn into a gas. The gas will rapidly expand shooting heated liquid out of the test tube like a cannon. ... Accidents happen even heating the test tube gently at an angle can result in heating liquid being shot out of the test tube
Explain why the electron configuration of 2-3-1 represents an atom in an excited state?
Answers
Answer:
See explanation
Explanation:
If we look at the electron configuration closely, we will discover that the element must have had a ground state electron configuration of 2,4.
This is because, the innermost shell usually holds two electrons while the outer shells hold eight electrons each. The four electrons must be accommodated in the second shell in the ground state configuration of the compound.
However, when the atom is excited, one electron from this shell may move to the third shell to give the excited state configuration 2-3-1 as shown in the question.
ammonium phosphate nh43po4 is an important ingredient in many fertilizers. it can be made by reacting phosphoric acid h3po4 with ammonia nh3. what mass of ammonium phosphate is produced by the reaction of 1.00g of phosphoric acid? be sure your answer has the correct number of significant digits.
Answers
When 4.9 g of phosphoric acid and 7.45 g of ammonia combine, 7.45 g (NH4)3PO4 of ammonium phosphate is created.
Ammonium phosphate is a crucial fertilizer, but why?Further assisting growth and root development is the supply of nitrogen to the surrounding soil, which is frequently accomplished using fertilizers containing ammonium phosphate. Because of its solubility, it can be quickly released into the soil, helping young plants and plants in need of revival.
What type of substance is ammonium phosphate, NH4 3po3?The inorganic chemical with the formula (NH4)3PO4 is called ammonium phosphate. It is the orthophosphoric acid ammonium salt.
To know more about ammonia visit:-
https://brainly.com/question/15409518
#SPJ4
The bending of waves due to a change is speed is called
Answers
Answer:
Refraction of waves involves a change in the direction of waves as they pass from one medium to another.
Explanation:
Refraction, or the bending of the path of the waves, is accompanied by a change in speed and wavelength of the waves.
Using the models of the molecules below, (H2 and CH4) what does it mean for a molecule to be symmetrical?
Answers
Answer:
A molecule is symmetrical if it can be cut into two identical halves.
Explanation:
H2 can be written as H + H
CH4 is not a symmetrical molecule
b Explain what would have happened if Jilly
had thrown the object with more force.
Answers
Answer:
force had thrown the object with more
Answer:
If an object is in motion and more force is applied to it, the object will begin moving faster
Explanation:
don't have any (sorry:[ )
which of the following element is not in group vii of the periodic table
Answers
Answer:
Oxygen group element, also called chalcogen, any of the six chemical elements making up Group 16 (VIa) of the periodic classification—namely, oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv). ... Modern version of the periodic table of the elements (printable).
Elemental analysis of a compound containing carbon, hydrogen, nitrogen, and oxygen gave the following mass percentages for each element: C: 42.36% H: 3.555% N: 16.47% O: 37.62% The molar mass of the compound is M
Answers
Elemental analysis of a compound containing carbon, hydrogen, nitrogen, and oxygen gave the following mass percentages for each element: C: 42.36% H: 3.555% N: 16.47% O: 37.62% The molar mass of the compound is Molecular formula = C6H14N2O2 Consider the total mass of the compound =100g Then mass of carbon = 49.30 g Mass of hydrogen = 9.653 g Mass of nitrogen = 19.16 g Mass of oxygen = 21.89 g Molar mass of carbon = 12.0 g/mol
What is molar mass ?In chemistry, the molar mass of a chemical compound is determined by dividing its mass by the molecular weight of the component that makes up the sample. The molar mass of a substance is a bulk attribute rather than a molecular one. The compound's molar mass is an average over numerous samples, which frequently have different masses because of isotopes. A terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth, the molar mass is most frequently calculated using the standard atomic weights. For converting between a substance's mass and amount in bulk amounts, the molar mass is the proper unit.
To learn more about molar mass from the given link:
https://brainly.com/question/22503632
#SPJ4
Does the diagram below demonstrate an endothermic or an exothermic reaction? Explain your reasoning.
PLEASE BE ACCURATE!!! Thank you so much!!:))
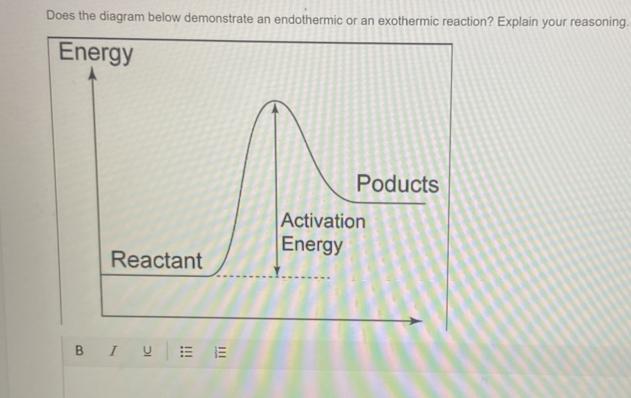
Answers
Answer:
Endothermic reaction.
Explanation:
The reactants are at a lower energy rate than the products. Because delta H is positive, energy is absorbed from the surroundings.
Why are some isotopes radioactive and some are not?
Answers
Answer: there are atoms that either have too many or too few neutrons or protons in their nuclei. This results in an imbalance between the jedi forces holding them together, which leads to an excess of internal energy. Such atoms are said to be unstable or radioactive.
Explanation:
Draw the dipole for PBr3
Answers
The dipole for PBr3 is shown in the attachment. PBr3 has non xero dipole moment because bromine atoms can not cancel out dipole moment of each other.
The three bromine atoms in this molecule do not cancel out the dipole moment of each other and molecule shows net dipole moment.it shows that PBr3 is a polar molecule.the partially positive charge surrounded by phosphorus atom and partially negative charged surrounded by bromine atom.
Dipole moment is used to define the symmetry of molecules. PBr3 has the trigonal pyramidal structure. bromine atom has more electronegative value than phosphorus.
Thus, the dipole moment in PBr3 is non zero due to unequal charge distribution.
To learn more about dipole moment here
https://brainly.com/question/24188648
#SPJ1

How can she test this idea in the Flowing Water Model?
Answers
She test this idea in the Flowing Water Model through integrated water flow model
Flowing water model is the computer programmed for simulating water flow through the integrated land surface water and groundwater flow systems and hydroelectric energy is power made by the moving water and water always flow downhills because of gravity and she She test this idea in the Flowing Water Model through integrated water flow model
Know more about model
https://brainly.com/question/17844323
#SPJ1
which are geologic evidence?
Answers
Why do halogens not form positive atoms?
Answers
Answer:
Due to the fact that halogens have an outer shell made up of seven valence electrons, they frequently obtain an additional electron and a negative charge.
Set up and solve a system of linear equations to balance the
following chemical reaction:
Limestone, CaCO3, neutralizes the acid, H3O, in acid rain by the
following unbalanced equation:
H3O + CaCO3 yields
→ H2O+Ca+CO2
Answers
A system of linear equations to balance the following chemical reaction:
Limestone, CaCO₃, neutralizes the acid, H₃ O, in acid rain is:
2H₃O + CaCO₃ → 3H₂O + Ca + CO₂
To balance the chemical equation:
H₃O + CaCO₃ → H₂O + Ca + CO₂
We need to ensure that the number of atoms of each element is the same on both sides of the equation.
Let's assign variables to the coefficients of each compound:
H₃O: x
CaCO₃: y
H₂O: z
Ca: a
CO₂: b
Now, we can set up the system of equations based on the number of atoms for each element:
For hydrogen (H):
3x = 2z
For oxygen (O):
3x + 3y = 2z
For calcium (Ca):
y = a
For carbon (C):
y = b
For calcium (Ca):
a = 1
Solving this system of equations will give us the balanced coefficients. Let's solve it:
From the equation y = a, we have y = 1.
From the equation y = b, we have b = 1.
Substituting b = 1 into the equation 3x + 3y = 2z, we have:
3x + 3 = 2z
From the equation 3x = 2z, we have x = (2/3)z.
Substituting x = (2/3)z into the equation 3x = 2z, we have:
3(2/3)z = 2z
2z = 2z
This equation is true for any value of z, indicating that z can take any value.
Therefore, we can choose z = 3 to simplify the coefficients:
x = (2/3)z = (2/3)(3) = 2
y = 1
z = 3
a = 1
b = 1
Thus, the balanced equation is:
2H₃O + CaCO₃ → 3H₂O + Ca + CO₂
To know more about linear equations here
https://brainly.com/question/32634451
#SPJ4
Given the following reaction at equilibrium, if Kc = 1.90 x 10^19 at 25.0 °C, Kp = ____.
H2 (g) + Br2 (g) = 2 HBr (g)
Answers
We need to find Kp for the given reaction at equilibrium with Kc = 1.90 x 10^19 at 25.0 °C. The reaction is:
H2 (g) + Br2 (g) ⇌ 2 HBr (g)
To convert Kc to Kp, we use the following formula:
Kp = Kc(RT)^(Δn)
Where:
Kp = Equilibrium constant in terms of pressure
Kc = Equilibrium constant in terms of concentration
R = Universal gas constant (0.08206 L atm / mol K)
T = Temperature in Kelvin
Δn = Change in the number of moles of gas (moles of products - moles of reactants)
First, convert the temperature from Celsius to Kelvin:
T = 25.0 °C + 273.15 = 298.15 K
Next, calculate Δn:
Δn = (2 moles of HBr) - (1 mole of H2 + 1 mole of Br2) = 2 - 2 = 0
Now, plug the values into the formula:
Kp = (1.90 x 10^19)(0.08206 L atm / mol K)(298.15 K)^0
Since Δn = 0, (RT)^(Δn) equals 1. Therefore, Kp equals Kc:
Kp = 1.90 x 10^19
So, for the given reaction at equilibrium, Kp is equal to 1.90 x 10^19.
TO KNOW MORE ABOUT reaction at equilibrium CLICK THIS LINK -
brainly.com/question/14575020
#SPJ11
Of these nonmetals, which one is likely to be the least reactive?
O oxygen (0)
O sulfur (S)
O chorine (CI)
O fluorine (F)
Answers
Answer:
Sulfur
Explanation:
Sulfur would be expected the be the least reactive as it has the least Electronegativity than the other non-metals on that list.
How many oxygen atoms are there in one molecule of CH3FO?
Answers
Explanation:
There should be 3 atoms in one molecule.
In a titration experiment, a 12.5 mL sample of 1.75 x 10^-2 M Ba(OH) 2 just neutralized 14.5 mL of HNO 3 solution. Calculate the molarity of the HNO 3 solution.
Answers
The number of moles must be equal, the ratio of the molarity of each must be equal to the ratio of the volumes used.
What is experiment?An experiment is a procedure or set of procedures used to test a hypothesis or explore a cause and effect relationship. It is a scientific method of investigation in which the investigator manipulates one or more independent variables and measures the subsequent effect on one or more dependent variables. Experiments are conducted to answer questions, test theories, and gain insight into cause-and-effect relationships.
The molarity of the HNO 3 solution can be calculated using the equation:
Molarity of HNO3 = (volume of HNO3 * molarity of Ba(OH)2) / volume of Ba(OH)2
Molarity of HNO3 = (14.5 mL * 1.75 x 10^-2 M) / 12.5 mL
Molarity of HNO3 = 1.4 x 10^-2 M
This equation works because the number of moles of HNO3 neutralized by the Ba(OH)2 must be equal to the number of moles of Ba(OH)2 used. The number of moles of each can be determined by multiplying the molarity of each by the volume used, and since the number of moles must be equal
To learn more about experiment
https://brainly.com/question/26150306
#SPJ4
What is the ph of a buffer solution that is 0. 172 m in hclo and 0. 131 m in naclo? hint: the ka of hclo is 3. 8 x 10-8
Answers
The pH of the mixture is 7.30 according to the equation.
What is pH?The pH measures the concentration of hydrogen ions in a solution as well as its acidity or alkalinity.
Normally, the pH scale runs from 0 to 14. Acidic aqueous solutions at 25 °C are those with a pH below 7, and basic or alkaline solutions are those with a pH above 7.
NaClO and HClO make up the buffer solution that is provided.
For the aforementioned buffer, HClO serves as our weak acid component while NaClO serves as our conjugate base component.
We will use the Henderson-Hasselbalch equation to determine the pH of the buffer solution, thus it is crucial to identify the weak acid and conjugate base component.
The conjugate base concentration and weak acid concentration of the buffer solution must be given in the equation.
The pKa value of the buffer solution must first be determined.
pKa = -log Ka
pKa = -log (3.8 * 10-8)
pKa = 7.42
The pH of the HClO-NaClO buffer solution can now be calculated using the Henderson-Hasselbalch equation.
pH = pKa + log[(conjugate base)(weak acid)]
pH = 7.42
pH = 7.42 + log[0.131M0.172M]
pH = 7.30
Hence,
The pH of the mixture is 7.30 according to the equation.
Learn more about pH here:
https://brainly.com/question/172153
#SPJ4
what is transition state
Answers
Answer:
transition state is a point in which electrons been removed from the atom
Answer:
The highest energy structure in the reaction coordinate.
Explanation:
in which type of reaction do Pb + O2 form PbO2?
synthesis
double displacement
single displacement
combustion
Answers
Answer: COMBUSTION
Explanation:
A combustion reaction is a reaction of a substance (in this case lead; Pb) with oxygen gas (O₂). It is usually an exothermic reaction that produces an oxide..
(need help asap)
How much of each reactant(zinc and hydrochloric acid) is needed to produce 150 grams of hydrogen gas
Answers
Explanation:
150 grams is .15 liters
1 mole of gas at STP occupies 22.414 liters
.15 liter of hydrogen represents .15/22.414 =
0.0066922 moles of H2
equation is
Zn + 2HCl → ZnCl2 + H2
1 mole of Zn will make 1 mole of H2
0.0066922 moles of Zn will make 0.0066922 moles of H2
To convert this molar quantity of zinc into grams we simply multiply by the atomic mass of zinc, which is
65.38 g/mol;-
Mass of Zn required = 0.0066922 moles * 65.38 g/mol = 0.4375390381 grams
https://www.quora.com/How-many-grams-of-Zinc-would-you-need-to-react-with-Hydrochloric-acid-to-produce-1-L-of-H2-gas
PLEASE HELP!!!!!!!!
The atom shown below is
An atom is shown with a large red central sphere and two levels of rings around it. The first ring has two small red spheres and the second has 8 small red spheres. The small spheres are evenly distributed on the rings.
A. likely to form a covalent bond
B. likely to form an ionic bond
C. not likely to form any bonds
D. likely to form double bonds
Answers
Consider the equation:S+3O2 → SO3
Is this equation balanced? Why or why not?
I will give brainlyiest to whoever writes the most detailed answer.!!!
Answers
On the left side of the equation, there is 1 atom of S and 6 atoms of O (3 O$_2$ molecules).
On the right side of the equation, there is 1 atom of S and 3 atoms of O (1 SO$_3$ molecule).
Therefore, to balance the equation, we need to add a coefficient of 2 in front of SO$_3$ to get:
S + 3O$_2$ $\rightarrow$ 2SO$_3$
Now, there are 2 atoms of S and 6 atoms of O on both sides of the equation.
__________________________________
S + 3O2 → SO3 = S + 3O2 → 2SO3= 2S + 302 → 2SO3= uS + v302 → wSO3= u = w = 6v = 3w= 6v/6 = 3w/6= v = w/2 = u = w= 2S + 3O2 → 3SO2No, The Equation Is Not Balanced.___________________________________
How are advancements in chemistry related to technology?
PLEASE BE ACCURATE! Thank you very much!:)
Answers
Advancements in chemistry have played a critical role in advancing technology in a number of ways such as ;
PharmaceuticalsEnergyNanotechnologyWhat is the relationship of advancements in chemistry to technology?Overall, advancements in chemistry are essential for developing new technologies and improving existing ones. By understanding the fundamental properties of materials and substances, chemists can create new materials with unique properties and develop new technologies that can improve our lives.
In term of Energy, Chemistry is crucial for developing new forms of energy, including renewable energy sources like solar, wind, and biofuels. Chemistry also plays a vital role in energy storage, such as the development of high-capacity batteries and fuel cells.
In term of Pharmaceuticals, Chemistry is essential for developing new drugs and improving existing ones. For example, the development of new cancer drugs has revolutionized cancer treatment, and new antibiotics have been developed to combat drug-resistant bacteria.
In term of Nanotechnology, Chemistry is critical for the development of nanotechnology, which involves working with materials at the nanoscale. Nanotechnology has many potential applications, including in electronics, medicine, and energy storage.
Learn more about technology at:
https://brainly.com/question/25110079
#SPJ1
How many grams of CO 2 are present in a container with a volume of 5.61 L if the gas exhibits a pressure of 5.66 atm at a temperature of 311 K?
Answers
Answer:
54.72 g
Explanation:
Mass = ?
Volume = 5.61 L
Pressure = 5.66 atm
Temperature = 311 K
The relationship between these equations is given by the ideal gas equation;
PV = nRT
where R = gas constant = 0.0821 atm L K-1 mol-1
n = PV / RT
n = 5.66 * 5.61 / (0.0821 * 311 )
n = 1.2436 mol
Number of moles = Mass / Molar mass
Mass = Number of moles * Molar mass = 1.2436 * 44 = 54.72 g
definition of all these plzz
Ulcers
Anemia
Diabetes
Anorexia
Bulimia
Cholesterol
Pacemaker
Endoscope
Stethoscope
Kidney dialysis
Protein
Carbohydrate
Fats
Fruits and vegetables
Answers
Answer:
I got 1 ulcers definition. an open score on an external