Answers
Related Questions
a gas has an initial volume of 3,480 mL and an initial temperature of - 70.0 C. what must be the temperature of the gas in kelvin if its volume is reduced to 2,450 mL
Answers
The temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
To determine the temperature of the gas in Kelvin after its volume is reduced, we can use the combined gas law, which relates the initial and final conditions of pressure, volume, and temperature for a given amount of gas.
The combined gas law equation is:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where P₁ and P₂ are the initial and final pressures, V₁ and V₂ are the initial and final volumes, T₁ is the initial temperature in Kelvin, and T₂ is the final temperature in Kelvin.
Given that the initial volume V₁ is 3,480 mL, the initial temperature T₁ is -70.0 °C (which needs to be converted to Kelvin), and the final volume V₂ is 2,450 mL, we can substitute these values into the equation.
To convert -70.0 °C to Kelvin, we add 273.15 to it, resulting in T₁ = 203.15 K.
Now we can solve for T₂:
(T₂ * V₁) / T₁ = V₂
T₂ = (V₂ * T₁) / V₁ = (2,450 mL * 203.15 K) / 3,480 mL
Simplifying the equation, we find:
T₂ ≈ 143.27 K
Therefore, the temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
In each of the following reactions identify an acid (if there is one) and then specify whether it is
an acid according to the Arrhenius definitions or the Bronsted-Lowry definitions or both.
a) H2CO3 + CN- HCN + HCO3-
b) F- + HSO4- HF + SO42-
c) HSO4- + H2O H3O+ + SO42-
Answers
a) In the reaction \(H_2CO_3 + CN^- = HCN + HCO^{3-}\), \(H_2CO_3\) acts as an acid by donating a proton (\(H^+\)) to \(CN^-\).
b) In the reaction \(F^- + HSO_4^{-} = HF + SO_4^{2-}\), \(HSO_4^{-}\) acts as an acid by donating a proton (\(H^+\)) to \(F^-\).
c) In the reaction \(HSO_4^- + H_2O = H_3O^+ + SO_4^{2-\), \(HSO_4^{-}\) acts as an acid by donating a proton (\(H^+\)) to \(H_2O\).
a) The acid is both an Arrhenius acid (produces \(H^+\) ions in water) and a Bronsted-Lowry acid (donates a proton to a base).
b) The acid is both an Arrhenius acid (produces \(H^+\) ions in water) and a Bronsted-Lowry acid (donates a proton to a base).
c) The acid is a Bronsted-Lowry acid (donates a proton to a base) but not an Arrhenius acid because it does not produce \(H^+\) ions in water. However, the \(H_3O^+\) ion that is formed can be considered an Arrhenius acid because it produces \(H^+\) ions in water.
For more question on proton click on
https://brainly.com/question/17351413
#SPJ11
Which energy output objects work with the solar panels?
Answers
Answer:
In this simulation, you will be able to “see” several different forms of energy and the changes(transfers) that can occur between them.You are also able to work with a system where you canmanipulate the energy input, observe the process of electrical energy generation and manipulatethe output.Click on the “Energy Systems” tab.We will do all of our work here. Be sure to clickthe “Energy Symbols” box so the different types of energy will be visible throughout the process
We have that for the Question "Which energy output objects work with the solar panels" it can be said that the energy output object that works with the solar panels is the
Inverter
From the question we are told
Which energy output objects work with the solar panels?
Generally
Inverter
A Inverter is a device used in a solar installation system,with its primary work being conversion from DC(Direct current) to AC(Alternating Current)
When the panel traps solar energy using photoelectric cells and transfers them to the Inverter converts the DC charge to AC in order to charge the batteryTherefore
the energy output object that works with the solar panels is the
Inverter
For more information on this visit
https://brainly.com/question/12534911?referrer=searchResults
Help me please.
How do animals see their pray without light?
Answers
Answer:Many nocturnal animals have a mirror-like layer, called the tapetum, behind the retina, which helps them make the most of small amounts of light.
Explanation:
what is the bond energy required to break one mole of carbon-carbon bonds
Answers
Answer:
100 kcal of bond energy
A 400 g sample is composed of 100 g of cesium (Cs) and 300 g of iodine (1).
What is the percent by mass of Cs in the sample?
A. 50.0%
B. 70.0%
C. 25.0%
D. 75.0%
Answers
The correct option C. 25.0%. The percentage by mass of cesium Cs in the sample is found as 25%.
Define the term mass percent equation?The ratio of the mass of the solute contained in a solution to the mass of a solution as a whole is known as the mass percent. This proportion must also be multiplied by 100 as indicated below since this kind of concentration, which would be generally determined for solid- or liquid-phase solutions, is represented in percentages..Mass percent = mass of solute/ mass of solution
A solution's total mass is equal to the product of the masses of a solute and solvent in which it contains since a solution consists of a solute and a solvent. Therefore, the mass percent of the a solution can also be determined using the following equation.mass percent = mass of solute/ mass of solution * 100 %
mass percent = 100/ 400 *100%
mass percent = 25%
Thus, the percentage by mass of cesium Cs in the sample is found as 25%.
To know more about the percent by mass, here
https://brainly.com/question/26150306
#SPJ1
Name 3 things in your home that involve chemical energy?
Answers
Batteries, natural gas, and coal are all chemical energy
An element is lustrous, brittle, and silver colored. What type of element is it
Metal
Metalloid
Non Metal
Other
Will give brainlist
Answers
What is the Rutherford experiment?
Answers
The Rutherford experiment rose above Thompson's Model 1911 with his gold-foil experiment. He designed an experiment to use the alpha particles created by a radioactive element as probes. Hope this helps you.
Titanium has an HCP unit cell for which the ratio of the lattice parameters c/a is 1.58. If the radius of the Ti atom is 0.1445 nm, (a) determine the unit cell volume, and (b) calculate the density of Ti and compare it with the literature value
Answers
The unit cell volume of the crystal is \(V_c = 9.9084*10^-^2^3 cm^3 / unit cell\) and the density of titanium is calculated as 4.71g/cm^3 while the literature value is 4.5 g/cm^3
Data;
radius = 0.1445 nmc/a = 1.58A = 46.88 g/molUnit Cell VolumeThe unit cell volume can be calculated as
\(V_c = 6R^2c\sqrt{3}\\\)
let's substitute the values into the formula
\(V_c = 6 * (1.445*10^-^8)^2 * 1.58 8 * a * \sqrt{3} \\a = 2R\\V_c = 6 * (1.445*10^-^8)^2 * 1.58* 2 * 1.445*10^-^8 * \sqrt{3}\\ V_c = 9.984*10^-^2^3 cm^3 / unit cell\)
The unit cell volume of the crystal is \(V_c = 9.9084*10^-^2^3 cm^3 / unit cell\)
Density of TiThe density of titanium can be calculated as
\(\rho = \frac{nA}{V_c N_a}\)
n = 6 for hcpA = 46.88 g/molNa = Avogadro's numberlet's substitute the values into the formula
\(\rho = \frac{nA}{V_c Na}\\ \rho = \frac{6*46.88}{9.9084*10^-^2^3* 6.023*10^2^3} \\\rho = 4.71 g/cm^3\)
The density of titanium is calculated as 4.71g/cm^3 while the literature value is 4.5 g/cm^3
Learn more on crystal lattice here;
https://brainly.com/question/6610542
State what could be used to keep the temperature of the conical flask and its
contents at a temperature of 45 °C throughout the reaction.
Answers
A water bath can be used to keep the temperature of the conical flask and its contents at a temperature .
What is a Water bath?This is a laboratory equipment which contains a heated source and is used to incubate samples in water at a constant temperature over a period of time.
This is why water bath will be best used to keep the temperature of the conical flask and its contents at a temperature of 45 °C throughout the reaction.
Read more about Water bath here https://brainly.com/question/24171510
How many moles of ammonium nitrate (NH4NO3) are present in 0.085 L of a 0.23 M ammonium nitrate (NH4NO3) solution?
Answers
Answer:
No. of moles \(\approx\) 0.020
Explanation:
To calculate the number of moles of a substance in a solution given its concentration and volume, we use the following formula:
\(\boxed{\mathrm{No. \ of \ moles = Concentration \times Volume}}\),
where concentration is in mol/dm³ (or M) and volume is in L.
In the question, we are told that the volume of the solution is 0.085 L and that its concentration is 0.23 M. Substituting these values into the formula above, we get:
No. of moles = 0.23 × 0.085
= 0.01955
\(\approx\) 0.020 mol (3 s.f.)
Therefore, there are 0.020 moles of NH₄NO₃ in the solution.
a student is trying to determine the heat of reaction for the acid-base neutralization reaction represented above. the student uses 0.50 m naoh and 0.50 m hcl solutions. which of the following situations, by itself, would most likely result in the least error in the calculated value of the heat of reaction?
Answers
Explanation:
Using a calorimeter with the highest possible accuracy to measure the heat of reaction.
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
A Sample of an Organic Compound Contain
0.624 Carbon, 0.065 hydrogen, 0·028 oxygen
(a) what is the Emperical formuler of the Compound.
(b) If the relative molecular mass of the Compound Is 1940 what is the moleculer
formular of the compound (C=12₁ H=1
N = 14,0= 16)
Answers
(a) The empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) The molecular formula of the compound is approximately \(C_{8}H_{118}O_{3}\)
To determine the empirical formula of the organic compound, we need to find the simplest whole-number ratio of the elements present.
(a) The given percentages of carbon, hydrogen, and oxygen can be converted into moles by dividing them by their respective atomic masses:
Carbon: 0.624 g / 12.01 g/mol = 0.052 mol
Hydrogen: 0.065 g / 1.008 g/mol = 0.064 mol
Oxygen: 0.028 g / 16.00 g/mol = 0.0018 mol
Next, we divide each of the mole values by the smallest mole value (0.0018 mol in this case) to obtain the mole ratio:
Carbon: 0.052 mol / 0.0018 mol ≈ 29
Hydrogen: 0.064 mol / 0.0018 mol ≈ 36
Oxygen: 0.0018 mol / 0.0018 mol = 1
Therefore, the empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) To find the molecular formula, we need the relative molecular mass of the compound, which is given as 1940 g/mol. The empirical formula mass can be calculated by summing the atomic masses in the empirical formula:
Empirical formula mass: (29 × 12.01 g/mol) + (36 × 1.008 g/mol) + (1 × 16.00 g/mol) = 588.94 g/mol
To find the multiplier, we divide the relative molecular mass by the empirical formula mass:
Multiplier: 1940 g/mol / 588.94 g/mol ≈ 3.29
Rounding to the nearest whole number, the molecular formula of the compound is approximately 3 times the empirical formula, resulting in \(C_{8}H_{118}O_{3}\).
In summary, the empirical formula of the compound is\(C_{29}H_ {36}O\), and the molecular formula is approximately \(C_{8}H_{118}O_{3}\).
Know more about empirical formula here:
https://brainly.com/question/1439914
#SPJ8
Please help me answer this question

Answers
What are the possible values of 1 and m for
n=4 ?
Answers
Answer:
If n = 4, then the possible values of 1 and m depend on the equation or expression being used. Without more information, it is impossible to determine what the possible values of 1 and m might be. Can you please provide more context or information about the problem you are trying to solve?
I NEED HELP ASAP !!!!

Answers
Answer:
5)A &D 6)B
Explanation:
Mass of Fe is 55.845 u
Mass of Cl is 35.453 u
And their sum is 91.298 u
How many particles are in 3.4 moles of NaCl?
Answers
Answer:
The answer is
2.049 × 10²⁴ particlesExplanation:
To find the number of entities or particles given the number of moles of a substance we use the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
n = 3.4 mol
We have
N = 3.4 × 6.02 × 10²³
We have the final answer as
2.049 × 10²⁴ particlesHope this helps you
How many moles are equal to 145g of zinc nitrate
Answers
Answer:
there are 6 MOLES of zinc nitrate in 145 g
The mass % of C in methane (CH4) is?
Answers
Answer:
74.87% Carbon
Explanation:
The molecular mass of CH4 is 16.042 g/ mole.
X=(100 × 12.01) / 16.042= 74.87%
Answer:
\(\boxed {\boxed {\sf 74.87 \% \ C}}\)
Explanation:
We want to find the mass percent composition of carbon in methane: CH₄
First, we must calculate the gram formula mass, also called the molar mass. Use the values for mass found on the Periodic Table. Look for carbon and hydrogen.
C: 12.011 gH: 1.008 gThere is no subscript after C, so there is just 1 atom. There is a subscript of 4 after H, so there are 4 atoms of hydrogen. We must multiply hydrogen's mass by 4.
C: 12.011 g H₄: 1.008 g * 4= 4.032 g CH₄= 12.011 g+ 4.032 g=16.043 gCalculate the percent composition.
\(\frac {mass \ of \ part}{mass \ of \ whole} *100\)
The part is the carbon, or 12.011 grams.
The whole is the entire compound, CH₄, or 16.043 grams.
\(\frac { 12.011 \ g }{ 16.043 \ g} *100\)
\(0.748675435*100\\74.8675435\)
Let's round to the nearest hundredth. The 7 in the thousandth place tells us to round the 6 to a 7.
\(74.87 \% \ C\)
The mass percent of carbon is 74.87%
Give two ways to make salt conduct.
Answers
Answer:
I think u willl get the answer fast in......
Answer:
this is because when a salt dissolves, its dissociated ions can move freely in solution, allowing a charge to flow. The resulting solution will conduct electricity because it contains ions.
Explanation:
What is the product of the reaction?
Please answer with the correct answer! Thanks.

Answers
Answer:
compound
Explanation:
it is calcium oxide, also known as quicklime
Which bonds form in the reaction shown in the diagram? 2 2H, + 0 H-H O=0 H-H → 2H 0 H-0-H H-OH A. The bonds between the two hydrogen atoms and between the two oxygen atoms B. The bonds between the two hydrogen atoms C. The bonds between the oxygen and hydrogen atoms D. The bonds between the two oxygen atoms

Answers
The water molecule is formed by the covalent bonding between oxygen and hydrogen atoms. Therefore, option C is correct.
What is covalent bonding ?Covalent bonding is a type of chemical bonding that involves the sharing of electrons between two atoms. In covalent bonding, the two atoms share a pair of electrons to fill their outermost electron shell and form a stable molecule.
This type of bonding usually occurs between non-metal atoms, which have a high electronegativity and tend to attract electrons strongly. In a covalent bond, the shared electrons are attracted to the positively charged nuclei of both atoms, creating a strong bond.
The strength of the bond depends on the number of shared electrons and the distance between the nuclei. Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the two atoms.
In water the bond is formed between oxygen atom and two hydrogen atoms. Hence, C is correct.
Find more on covalent bonding:
https://brainly.com/question/12661797
#SPJ7
Calculate ΔHrxn for the following reaction: Al2O3(s)+3CO(g)→2Al(s)+3CO2(g) Use the following reactions and given ΔH values: 2Al(s)+32O2(g)→Al2O3(s),ΔH CO(g)+12O2(g)→CO2(g),ΔH==−1675.7kJ−282.7kJ
Answers
The desired reaction is 2Al(s) + 3CO2 from Al2O3(s) + 3CO(g) (g) The reactions include 2 Al(s), 3/2 O2(g), and Al2O3(s), with H = 1675.7kJ. ————————— (1) CO(g) = CO2 + 1/2 O2(g) (g).
How is H inside a calculated?As a result, the enthalpies of a reactants and products are added together, and the result is used to compute the enthalpy of a reaction. This endothermic process generates and absorbs environmental heat if H is positive. This reaction is exothermic so emits heat into the environment if H is negative.
What is the H heat?A negative H indicates that heat is transferred from the a system towards its surroundings, whereas a positive H indicates that heat is transferred from the surroundings into the system. An enthalpy of reaction (Hrxn) for a chemical reaction is the difference of enthalpy between the products and reactants; Hrxn is measured in kilojoules per mole.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
and Neutron
4. In general, which of the following statements about metals is true?
A. Metals need to be stored in sealed containers for safety.
B. Metals are malleable, ductile, and can carry an electric current.
C. Metals are highly reactive substances.
D. Metals do not react with oxygen.
|

Answers
Answer:
B. Metals are malleable, ductile, and can carry an electric current.
Explanation:
A: Light
B: Sound
C: Heat
D: Motion
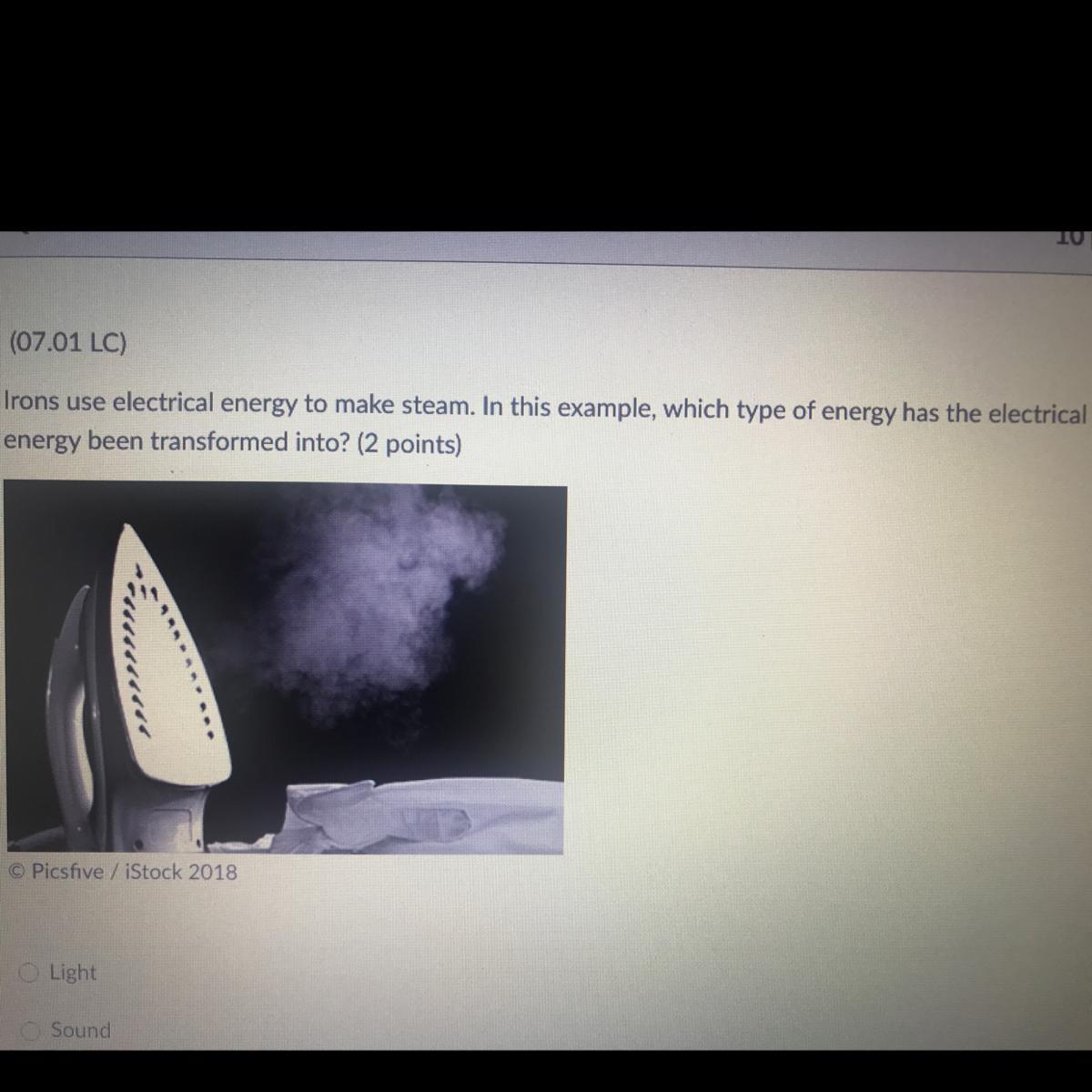
Answers
According to the article, why is deep-sea mineral extraction a controversial practice?
Answers
According to the article, deep-sea mineral extraction a controversial practice because it destruct the natural land wildlife
Deep sea mining is the process of retrieving mineral deposited from deep seabed and there are many environmental problems created by deep ocean mines which level the ocean floor to extract the minerals and the most direct impacts at mining sites are destruction of natural land forms and the wildlife they host compaction of the sea floor and creation of sediment plumes that disrupt aquatic life and deep sea contain many different resources such as silver, gold, copper, manganese, cobalt, and zinc
Know more about extraction
https://brainly.com/question/28600760
#SPJ1
What mass (grams) of antimony(III) chloride would be produced by reacting with 112 liters of chlorine measured at STP?
Answers
Answer:
radius = 16 in ; height = 27 in
what is the first element in the periodic table?
Answers
Answer:
the first element on the table is Hydrogen