Josh decided to investigate the effect of the reagents listed in part b of the lab on the cobalt chloride system described on page 60 of the lab manual. Their cobalt chloride solution composed of 0. 20 m cocl2 in 4. 0 m nacl. Identify one reaction they would have observed that to push the reaction either in the forward or reverse direction by filling in the blanks in the statement given below. Identify what phenomenon occurred that caused the equilibrium to shift.
Answers
One of the reactions they would have observed that would push the reaction either in the forward or reverse direction is NaCl (aq) ⇌ Na+ (aq) + Cl- (aq)
Cobalt chloride system described on page 60 of the lab manual consist of cobalt chloride solution containing 0.20 M CoCl2 in 4.0 M NaCl. When one of the reagents listed in part b of the lab is added, there will be a change in the reaction system and this will push the reaction either in the forward or reverse direction.Reaction that would push the reaction either in the forward or reverse direction by filling in the blanks in the statement given below is shown below;NaCl (aq) ⇌ Na+ (aq) + Cl- (aq)When a small quantity of NaCl is added to the Cobalt chloride solution, the concentration of Cl- ions increases. According to Le Chatelier's principle, the equilibrium will shift towards the left in order to counteract the increase in Cl- ions. This is because by reducing the amount of Cl-, the equilibrium system will try to balance the added NaCl. So, the phenomenon that occurred that caused the equilibrium to shift was the addition of NaCl to the cobalt chloride solution.
Therefore, we can say that the addition of NaCl to the cobalt chloride system described on page 60 of the lab manual will cause the equilibrium to shift towards the left.
To know more about Le Chatelier's principle, click here
https://brainly.com/question/29009512
#SPJ11
Related Questions
6. would you expect wet soil to have the same angle of repose as dry soil? why or why not?
Answers
Wet soil would not have the same angle of repose as dry soil. The angle of repose is the maximum angle at which a granular material can remain stable without collapsing. Wet soil tends to stick together due to the presence of water, which increases the cohesion between soil particles.
The angle of repose refers to the maximum angle at which a material can be piled up without sliding down. Wet soil is more cohesive than dry soil, which means that the particles are more likely to stick together, resulting in a higher angle of repose. Therefore, wet soil would not have the same angle of repose as dry soil. The angle of repose for wet soil is typically higher than that of dry soil, making it more stable and less prone to sliding.
However, the angle of repose may vary depending on the type of soil and its moisture content.
To know something about the angle of repose, click below.
https://brainly.com/question/30884145
#SPJ11
Explain how surface waves work.
Answers
Which situation provides the best evidence that a chemical reaction is taking place?
Answers
Answer: The following can indicate that a chemical change has taken place, although this evidence is not conclusive: Change of odor.
Explanation:
If the metal from problem 4 was initially at room temperature (22 0 C), what would the final temperature of the metal be? You know that you add 120 joules of energy to the metal. What change in temperature would you observe Q = is energy as Heat, 120 Joulesm = mass in grams, 5.0 gramsc = is the specific heat capacity, 0.385 J/g°CΔT = the change in temperature, calculated as Final Temperature - Initial T120 = 5 * 0.385 * T120 = 1.925TT = 62°C of change in temperature
Answers
The question is mostly solved. The definition of heat is used for this problem which tells us:
\(Q=mCp\Delta T\)Where,
Q is the heat added to the system, 120 J
m is the mass of the metal, 5.0 g
Cp is the specific heat of the metal, 0.385J/g°C
dT is the change of temperature:
\(\Delta T=T_2-T_1\)T2 is the final temperature, unknown
T1 is the initial temperature, 22°C
We clear the final temperature from the equation:
\(\begin{gathered} Q=mCp(T_2-T_1) \\ Q=mCpT_2-mCpT_1 \\ T_2=\frac{Q+mCpT_1}{mCp} \end{gathered}\)Now, we replace the known data:
\(T_2=\frac{120J+5.0g\times0.385\frac{J}{g\degree C}\times22\degree C}{5.0g\times0.385\frac{J}{g\degree C}}\)\(\begin{gathered} T_2=\frac{120+5.0\times0.385\times22}{5.0\times0.385}\degree C \\ T_2=84\degree C \end{gathered}\)Answer:
The final temperature of the metal will be 84°C
The change in the temperature will be 84°C-22°C=62°C
What is the half life of 44ti if a 1.0ng sample decays at the rate of 4.3*108 disintegration?
Answers
The half-life of 44ti can be calculated using the given information. The rate of decay is \(4.3*10^8\) disintegrations, and the initial sample size is 1.0 ng.
For finding the half-life, we can use the formula:
half-life = (ln(2)) / (decay constant)
First, we need to find the decay constant using the given rate of decay:
decay constant = rate of decay / initial sample size
decay constant = (4.3*10^8 disintegrations) / (1.0 ng)
Now, we can substitute the decay constant into the half-life formula:
half-life = ln(2) / (decay constant)
half-life = ln(2) / [(\(4.3*10^8\) disintegrations) / (1.0 ng)]
So, the half-life of 44ti is ln(2) / [(\(4.3*10^8\) disintegrations) / (1.0 ng)].
To know more about radioactive decay refer here:
https://brainly.com/question/1770619?#
#SPJ11
Identify the bacteria that are responsible for turning nitrogen from the atmosphere into usable nitrogen for plants.
Answers
Rhizobium bacteria were soil microbes that have the ability to convert dissolved nitrogen from the atmosphere. These root nodules on woody plants contain rhizobium bacteria.
What kind of bacteria releases nitrogen into the air?The nitrogen cycle is finished by denitrification, which turns nitrate (NO3-) back into gaseous nitrogen (N2). The driving force behind this procedure is denitrifying microorganisms. These bacteria generate energy from nitrate rather than oxygen, which results in the release of nitrogen gas into the environment.
What three kinds of bacteria utilise nitrogen?Azotobacter, Bacillus, Clostridium, or Klebsiella species are a few instances that belong to this category of nitrogen-fixing bacteria. These organisms must acquire their own energy source, as was previously said. Typically, they do this by oxidising organic molecules generated by the other organisms or as a result of decomposition.
To know more about nitrogen visit:
https://brainly.com/question/16711904
#SPJ1
can you guys please fact check me I don't wanna get a bad grade and please tell me if any are wrong 100 points and don't just take them





Answers
Answer:
1. the reaction rates can not decrease
2. correct!
3. correct!
4. decreasing the amount of reactants
5. correct!
Explanation:
good luck!
How is Earth different from other planets?
OA. It has liquid water.
OB. It has mountains.
OC. It has a moon.
OD. It gets light from the Sun
Answers
Explanation: Earth is the only planet (as of now) that has organic life, and has liquid water on the surface.
Hope this has helped you!
1. Determine the molecular formula of an oxide of iron in which the mass of iron and oxygen are 69.9% and 30% respectively given that the molar mass of the oxide 159.898/mol, find the empirical and molecular formula.
2. a crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%.
3. In three moles of Ethane calculate the following
1. calculate number of carbon atoms.
2. number of moles of hydrogen atoms
3. number of molecules of Ethane.
Answers
1a. The empirical formula of the compound is Fe₂O₃
1b. The molecular formula of the compound is Fe₂O₃
2a. The molecular formula of the anhydrous salt is MgSO₄
2b. The formula of the crystalline salt is MgSO₄.7H₂O
3i. The number of mole of carbon atoms in the compound is 6 moles
3ii. The number of mole of hydrogen atoms in the compound is 18 moles
3iii. The number of molecules in 3 moles of ethane is 1.806×10²⁴ molecules
1a. How to determine the empirical formulaFe = 69.9%O = 30%Empirical formula =?Divide by their molar mass
Fe = 69.9 / 56 = 1.248
O = 30 / 16 = 1.875
Divide by the smallest
Fe = 1.248 / 1.248 = 1
O = 1.875 / 1.248 = 3/2
Multiply by 2 to express in whole number
Fe = 1 × 2 = 2
O = 3/2 × 2 = 3
Thus, the empirical formula of the compound is Fe₂O₃
1b. How to determine the molecular formulaEmpirical formula = Fe₂O₃Molar mass of compound = 159.89 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[Fe₂O₃]n = 159.89
[(56×2) + (16×3)]n = 159.89
160n = 159.89
n = 159.89 / 160
n = 1
Molecular formula = [Fe₂O₃]n
Molecular formula = [Fe₂O₃] × 1
Molecular formula = Fe₂O₃
2a. How to determine the molecual formula of the anhydrous saltWe'll begin by calculating the empirical formula
Mg = 20.0% S = 26.66% O = 53.33%Empirical formula =?Divide by their molar mass
Mg = 20.0 / 24 = 0.83
S = 26.66 / 32 = 0.83
O = 53.33 / 16 = 3.33
Divide by the smallest
Mg = 0.83 / 0.83 = 1
S = 0.83 / 0.83 = 1
O = 3.33 / 0.83 = 4
Thus, the empirical formula of the anhydrous salt is MgSO₄
The molecular formula of the anhydrous salt can be obtained as follow:
Empirical formula = MgSO₄Molar mass of compound = 120 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[MgSO₄]n = 120
[24 + 32 + (16×4)]n = 159.89
120n = 120
n = 120 / 120
n = 1
Molecular formula = [MgSO₄]n
Molecular formula = [MgSO₄] × 1
Molecular formula = MgSO₄
2b. How to determine the formula of the crystalline saltWater (H₂O) = 51.2%Anhydrous salt (MgSO₄) = 100 - 51.2 = 48.8%Formula of crystalline salt =?Divide by their molar mass
MgSO₄ = 48.8 / 120 = 0.4
H₂O = 51.2 / 18 = 2.8
Divide by the smallest
MgSO₄ = 0.4 / 0.4 = 1
H₂O = 2.8 / 0.4 = 7
Thus, the formula of the crystalline salt is MgSO₄.7H₂O
3i. How to determine the mole of carbon atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 2 moles of carbon atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 2 = 6 moles of carbon atoms
3ii. How to determine the mole of hydrogen atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 6 moles of hydrogen atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 6 = 18 moles of hydrogen atoms
3iii. How to determine the number of moleculesFrom Avogadro's hypothesis,
1 mole of ethane = 6.02×10²³ molecules
Therefore,
3 moles of ethane = 3 × 6.02×10²³ molecules
3 moles of ethane = 1.806×10²⁴ molecules
Learn more about empirical formula:
https://brainly.com/question/24297883
Learn more about Avogadro's number:
https://brainly.com/question/26141731
#SPJ1
Complete question
2. A crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%. Ccalculate the molecular formula of the anhydrous and the crystalline salt. The molecular weight of the anhydrous salt is 120
Which contributes to the dissolution of sugar in water
Answers
Answer:
Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent.
Solid aluminum has a specific heat capacity of 0.90 J/ gxK. How many joules of heat are absorbed to raise the temperature of 24.0 grams of aluminum from 300. K to 350. K?
1. 22 J
2. 45 J
3. 1100 J
4. 1200 J
Answers
Answer:: 1100 j
Explanation:
Solid aluminum has a specific heat capacity of 0.90 J/ gK joules of heat are absorbed to raise the temperature of 24.0 grams of aluminum from 300. K to 350. K is 11.080 J
Heat is the transfer of kinetic energy from one medium or object to another from an energy source to a medium or object
Here given data is
Specific heat capacity = 0.90 J/gK
Temperature = 300. K to 350. K = 350 - 300 = 50 K
Mass = 24.0 grams
We have to calculate the heat = ?
Q =mCΔT
Q = 24.0 grams× 0.90×50 K
Q = 11.080 J
11.080 J heat are absorbed to raise the temperature
Know more about heat
https://brainly.com/question/22406880
#SPJ1
What mass of kcl in grams must be added to 500 ml of a 0. 15 m kcl solution to produce a 0. 40 m solution.
Answers
91.4 grams mass of KCl is required.
Concentration = moles/Volume
2.45M=mol/0.5L
2.45M⋅0.5L=mol
mol=1.225
Convert no. of moles to grams using the atomic mass of K + Cl
1.225mol⋅(39.1+35.5)/gmol
1.225mol⋅74.6g
mol=1.225⋅74.6g = 91.4g
What is Concentration?The formula for determining concentration from moles and volume is pretty straightforward. Simply divide the volume of solution by the moles of solute.The number of moles of a component divided by the sum of the moles in the solution is what is known as a mole fraction, which is a unit of concentration. Mole fraction is a unit-free expression because it represents a ratio.To learn more about concentrations, refer to the given link:
https://brainly.com/question/23312431
#SPJ4
use the mo diagrams to calculate the bond order for li2 and li2− . express the bond order for li2 followed by the bond order for li2− separated by a comma.
Answers
the bond order for Li2 is 1, and the bond order for Li2- is -0.5.To calculate the bond order for Li2 and Li2-, we need to construct their molecular orbital (MO) diagrams.
For Li2, each Li atom contributes one valence electron. In the MO diagram, we have two Li 1s orbitals that combine to form two molecular orbitals: one bonding (σ) and one antibonding (σ*). Since there are two electrons in the bonding molecular orbital and no electrons in the antibonding molecular orbital, the bond order for Li2 is (2-0)/2 = 1.
For Li2-, we have an additional electron, resulting in a total of three valence electrons. In the MO diagram, one electron occupies the bonding molecular orbital (σ) and the other two occupy the antibonding molecular orbital (σ*). Therefore, the bond order for Li2- is (1-2)/2 = -0.5.
So, the bond order for Li2 is 1, and the bond order for Li2- is -0.5.
To learn more about bond click here: brainly.com/question/30508122
#SPJ11
How to handle coin top containers ?- place hand over label- place stopper on workbench to avoid dropping it- pour using a stirring rod- wipe off any drops
Answers
To handle coin top containers place hand over label, place stopper on workbench to avoid dropping it, pour using a stirring rod and wipe off any drops.
Handling coin top containers requires care to prevent spills and ensure safe handling. Here are the steps to handle coin top containers properly:
1. Place hand over the label: Before handling the coin top container, ensure that your hand is securely placed over the label or lid. This will help prevent accidental opening or spillage of the container's contents.
2. Place stopper on workbench: If the coin top container has a removable stopper, place it on a stable workbench or surface. By doing so, you can keep the stopper readily accessible and avoid the risk of dropping it or misplacing it.
3. Pour using a stirring rod: If you need to transfer the contents of the coin top container, use a clean stirring rod or suitable utensil for pouring. Slowly and carefully pour the desired amount of liquid from the container, taking care to control the flow and minimize the chances of spills or splashes.
4. Wipe off any drops: After pouring, inspect the outside of the container and the surrounding area for any spills or drops. If you notice any liquid on the container or workbench, use a clean cloth or paper towel to wipe off the drops promptly. This helps maintain cleanliness and prevent accidental contact with the spilled substance.
Remember to follow any specific handling instructions provided by the manufacturer or any safety guidelines relevant to the contents of the coin top container.
Learn more about coin top containers at: https://brainly.com/question/12090900
#SPJ11
List two ways in which enzymatic browning can be prevented?
Answers
Adding citric and irradiation
Hope it helps.
in glycolysis, atp is consumed in the reaction producing which compound?
Answers
In glycolysis, ATP is consumed in the reaction producing compound is adenosine diphosphate (ADP)
Glycolysis is the initial stage of cellular respiration in which glucose is broken down into pyruvate. In the cytoplasm of the cell, this process happens. Two molecules of ATP are used in glycolysis, and four are produced. The product of glycolysis is pyruvate, which can enter either anaerobic or aerobic cellular respiration.
Adenosine triphosphate (ATP) is a type of nucleotide that is made up of adenine, ribose, and three phosphate groups. ATP is a universal energy currency in organisms that use oxygen, storing and delivering energy for cellular processes. During cellular respiration, ATP is synthesized and consumed repeatedly. ATP is produced in the later stages of cellular respiration, including oxidative phosphorylation and chemiosmosis.
Learn more about glycolysis at:
https://brainly.com/question/26990754
#SPJ11
Which of the following best describes a function of the nervous system as
discussed in class?
to store urine
O to move limbs
to receive stimuli from inside and outside the body
to take in oxygen and transport it to the cells
Answers
Answer: The answer is to receive stimuli from inside and outside the body.
Explanation:
Which element listed below is a nonmetal?
O A. sodium (Na)
O B. magnesium (Mg)
O C. iron (Fe)
OD. chlorine (CI)
Answers
Answer:
D: Chlorine( I may be wrong my chemistry year was a nightmare)
The awnser is chlorine (Cl) trust me
What are the coefficients when this equation is balanced explain
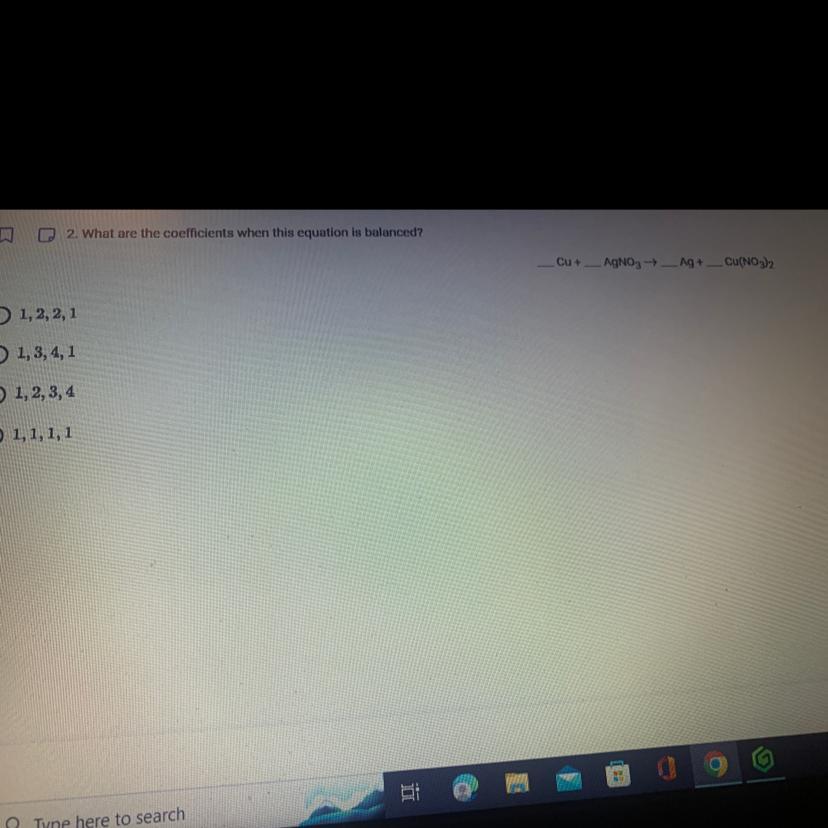
Answers
The balanced equation will be:
Cu + 2 AgNO3 ----> 2Ag + Cu(NO3)2
The balancing of equation is done to satisfy the law of conservation of mass. This law states - "mass can neither be created, nor be destroyed". Thus, the mass of the element in the equation cannot be created or destroyed, it is just shifted.
The answer is 1,2,2,1; the first option.
What is an example of a solid turning into another state of matter?
Answers
Answer:
Explanation:
Let me start with a simple one:
An ice cube ( solid ) changing into water ( a liquid )
Another example:
Dry ice ( solid ) submiling.
HOPE IT HELPES
The mass of a chlorine atom in atomic mass units is approximately 35. The atom has 18 neutrons (true or false)
Answers
The given statement "The mass of a chlorine atom in atomic mass units is approximately 35. The atom has 18 neutrons " is False because The mass number of a chlorine atom is approximately 35 (rounded to the nearest integer), not its atomic mass in atomic mass units.
The atomic mass of chlorine in atomic mass units is about 35.5. This is because the chlorine atom has two isotopes, chlorine-35 and chlorine-37, with atomic masses of approximately 35 and 37, respectively. The atomic mass of an element in atomic mass units is a weighted average of the atomic masses of its isotopes, taking into account their natural abundances.
Therefore, if a chlorine atom has a mass number of 35, it must have 17 neutrons (since the mass number = number of protons + number of neutrons).
For more question on atomic mass click on
https://brainly.com/question/30390726
#SPJ11
Which substance should have its atmospheric concentrations reduced in order to return acid rain to rain's normal ph?.
Answers
To restore acid rain to its natural pH, Sulphur should be present in lower proportions in the atmosphere. Sulphur dioxide emissions mixed with nitrogen oxide emissions cause acid rain.
Both substances interact with airborne molecules including water, oxygen, and other molecules to produce this effect. Particularly when it comes to aquatic life, this phenomena is exceedingly hazardous for both people and other animals and plants. For example, the elevated pH prevents several kinds of freshwater fish from developing or growing. When the pH is even lower, however, in some situations, the organisms are unable to survive and eventually perish. Therefore, a decrease in atmospheric Sulphur concentrations could lessen acid rain and bring rain back to its normal pH in the atmosphere.
Learn more about Sulphur here
https://brainly.com/question/23535995
#SPJ4
For which properties can a student design an investigation of the electrostatic forces between molecules in a substance?.
Answers
Intermolecular forces such as dipole-dipole forces, London dispersion forces exist between molecules and these depend on strength of electronegativity of molecule. For knowing melting point, boiling point, surface tension, and vapor pressure properties, student design an investigation of the electrostatic forces between molecules in a substance.
What is intermolecular forces of attraction?Intermolecular forces of attraction is force of attraction that make two molecule come closer. Intermolecular forces of attraction is directly proportional to the electronegativity of the molecule.
Size of electronegative molecule is small so, if any other molecule or molecule come into contact with this molecule then this molecule attract the electron of other molecule very efficiently and therefore the force between the molecule increases. Electrostatic forces between molecules in a substance helps in knowing melting point, boiling point, surface tension, and vapor pressure properties
Therefore, for knowing melting point, boiling point, surface tension, and vapor pressure properties, student design an investigation of the electrostatic forces between molecules in a substance.
To learn more about intermolecular forces of attraction, here:
https://brainly.com/question/26701678
#SPJ1
g what is the angle between the carbon-hydrogen bond and the carbon-nitrogen bond in the hydrogen cyanide ( ) molecule?
Answers
The angle between the carbon-hydrogen bond and the carbon-nitrogen bond in the hydrogen cyanide ( ) molecule is 180 degrees
The HCN Lewis structure comprises three different atoms: hydrogen, carbon, and nitrogen. It is a polar molecule with a bond angle of 180 degrees.Hydrogen Cyanide (HCN) is a colorless, flammable, and poisonous liquid.
In the HCN Lewis structure, there is a triple bond between carbon and nitrogen and a single bond between C and H.A solution of hydrogen cyanide in water is called hydrocyanic acid. There are two sigma bonds, C-H and C-N.The bond between the C and the N in hydrogen cyanide is a triple bond.The hybrid orbital is sp, due to the linear geometry of the molecule.
Find out more about HCN
brainly.com/question/21304911?referrer=searchResults
#SPJ4
When steel and zinc were connected, which one was the cathode?
Steel
Zinc
☐ neither
both
Answers
When steel and zinc were connected, zinc is the cathode. The term cathode refers to the electrode that is reduced during an electrochemical reaction.
The electrons are moved from the anode to the cathode during an electrochemical reaction in order to maintain a current in the wire that links the two electrodes.
According to the galvanic series, zinc is more active than iron, meaning that it is more likely to lose electrons and be oxidized. As a result, when steel and zinc are connected, zinc will act as the anode and lose electrons, whereas iron (steel) will act as the cathode and receive the electrons transferred by zinc.
To know more about electrochemical reaction visit:-
https://brainly.com/question/13062424
#SPJ11
Why does anhydrous aluminium chloride have lower melting point than anhydrous aluminium fluoride?
Answers
Answer:
Explanation:
Molecule of aluminium fluoride is more polar than molecule of aluminium chloride . It is so because fluorine is more electronegative than chlorine . On Pauling scale , the electronegativity of fluorine is 3.98 whereas that of chlorine is 3.16 . Hence in aluminium fluoride , there is stronger inter molecular bonding because of more polar nature of molecules . In aluminium chloride , there is weaker inter molecular bonding because of comparatively polar nature of molecules . Hence melting point of anhydrous aluminium fluoride is higher than that of anhydrous aluminium chloride .
How many significant figures does this measurement contain? 0.0000508800 Gm
Answers
Answer:
There are 6 significant figures
Answer:
6 significant figures
Explanation:
When counting significant figures, start on the left with the first non-zero number. Count all numbers that fall between non-zero numbers. Count all zeros after a 1-9 and after the decimal.
Explain how the skeletal and muscular system work together to support body movement from this picture.

Answers
Answer:
Tendons connect the skeletal system to the muscular system by attaching muscle to bone. When muscle contracts, the tendon acts on the bone, causing movement. Joints, the point at which two or more bones connect, can be fixed, slightly movable, or freely movable.
One atom has 11 protons and 13 neutrons. Another has 11 protons and 12 neutrons. Are they the same or different elements?
Answers
Answer:
deferent elements
Explanation:
because 11n 12 is not the same as well as13
Answer: They are the same elements
Explanation:
They're same elements because the number of electrons are same (number of protons=electrons).
The number neutrons don't matter.
If any two atoms have the same number of Electrons and protons and has different number of neutron's they still are the same element.
calculate the ml of 0.553 m hydrochloric acid needed to completely react with 85.0 g of a 5.00% lead (ii) nitrate solution. 2 hcl(aq) pb(no3)2(aq) --> pbcl2(s) 2 hno3(aq)
Answers
Approximately 46.0 mL of 0.553 M HCl is needed to completely react with 85.0 g of a 5.00% lead (II) nitrate solution.
To calculate the volume of 0.553 M hydrochloric acid (HCl) needed to react with 85.0 g of a 5.00% lead (II) nitrate (Pb(NO₃)₂) solution, we need to follow a series of steps:
Step 1: Calculate the amount of lead (II) nitrate (Pb(NO₃)₂) in the solution.
Given:
Mass of (Pb(NO₃)₂) solution = 85.0 g
Percentage concentration of (Pb(NO₃)₂) = 5.00%
First, we need to convert the percentage concentration to grams of (Pb(NO₃)₂):
Mass of (Pb(NO₃)₂) = (Percentage concentration / 100) x Mass of solution
Mass of (Pb(NO₃)₂) = (5.00 / 100) x 85.0 g
Mass of (Pb(NO₃)₂) = 4.25 g
Step 2: Calculate the number of moles of (Pb(NO₃)₂).
To do this, we need to divide the mass of (Pb(NO₃)₂) by its molar mass.
The molar mass of (Pb(NO₃)₂) = 207.2 g/mol (lead atomic mass: 207.2 g/mol, nitrogen atomic mass: 14.0 g/mol, oxygen atomic mass: 16.0 g/mol)
Number of moles of (Pb(NO₃)₂) = Mass of (Pb(NO₃)₂) / Molar mass of (Pb(NO₃)₂)
Number of moles of (Pb(NO₃)₂) = 4.25 g / 331.2 g/mol
Number of moles of (Pb(NO₃)₂) = 0.0128 mol
Step 3: Use the stoichiometry of the balanced chemical equation to determine the number of moles of HCl required.
From the balanced equation: 2 HCl(aq) + Pb(NO₃)₂ (aq) → PbCl₂(s) + 2 HNO₃(aq)
The stoichiometric ratio between Pb(NO₃)₂ and HCl is 1:2.
Number of moles of HCl = 2 x Number of moles of Pb(NO₃)₂
Number of moles of HCl = 2 x 0.0128 mol
Number of moles of HCl = 0.0256 mol
Step 4: Calculate the volume of 0.553 M HCl using the number of moles of HCl.
To find the volume of the HCl solution, we can use the formula:
Volume (L) = Number of moles / Concentration (M)
Volume of HCl = Number of moles of HCl / Concentration of HCl
Volume of HCl = 0.0256 mol / 0.553 mol/L
Volume of HCl = 0.046 L
Finally, convert the volume from liters to milliliters:
Volume of HCl = 0.046 L x 1000 mL/L
Volume of HCl = 46.0 mL
Therefore, approximately 46.0 mL of 0.553 M HCl is needed to completely react with 85.0 g of a 5.00% lead (II) nitrate solution.
To know more about stoichiometry, refer to the link below:
https://brainly.com/question/28780091#
#SPJ11