It takes much less energy to change the temperature of oil than it does to change the temperature of water
Answers
Answer:
i think so
Explanation:
Related Questions
Air pressure is caused by: gas particles striking the walls of a container or an object. b. gas particles are taking a test gas particles reaching 0 degrees Kelvin d. Air pressure has nothing to do with gas.
Answers
Answer:
D
Explanation:
WILL MARK BRAINLIEST PLZ HELP !!!!!!!!!!!
Which method would be best for separating the components of a mixture that is made from two different liquids?
A)distillation
B)evaporation
C)filtration
D)sorting
Answers
Answer: A)
The best method for separating two liquids in a mixture is distillationamong the choices. The best method for separating two liquids in a
mixture is distillation among the choices. Distillation separates liquids in
terms of their volatility
Write the balanced equation for the reaction of 1 mol of naoh with 1 mol of h2po4
Answers
Answer:
3 NaOH + H₃PO₄ -----> Na₃PO₄ + 3 H₂O
Explanation:
This appears to be a double-displacement reaction. In these reactions, the cation of one compound is swapped with the cation of another. Therefore, Na⁺ (from NaOH) is swapped with H⁺ (from H₃PO₄). When constructing the new compounds, you may need to alter the amount of each ion within a particular compound to to make it neutral.
New Product #1: Na₃PO₄
----------> Na⁺ and PO₄³⁻
----------> +1 + 1 + 1 + (-3) = 0
New Product #2: H₂O (or HOH)
----------> H⁺ and OH⁻
----------> +1 + (-1) = 0
Now that you know the products, you need to balance the chemical equation. An equation is balanced when there is an equal amount of each element on both sides. These amounts can be modified by adding coefficients.
The unbalanced equation:
NaOH + H₃PO₄ -----> Na₃PO₄ + H₂O
Reactants: 1 sodium, 5 oxygen, 4 hydrogen, 1 phosphorus
Products: 3 sodium, 5 oxygen, 2 hydrogen, 1 phosphorus
The balanced equation:
3 NaOH + H₃PO₄ -----> Na₃PO₄ + 3 H₂O
Reactants: 3 sodium, 7 oxygen, 6 hydrogen, 1 phosphorus
Products: 3 sodium, 7 oxygen, 6 hydrogen, 1 phosphorus
**I was having trouble balancing the equation with the reactant H₂PO₄⁻. From my experience, I believe this compound may have been mistyped. As such, I used H₃PO₄. Please let me know if the reactant was written properly**
Part 1: Name two elements that have the same properties as magnesium (Mg). (4 points)
Part 2: Determine the number of protons, electrons, and neutrons present in an atom of potassium (K). Explain how you determined your answer using complete sentences. (6 points)

Answers
Two elements having same properties as magnesium are calcium and strontium. The number of protons and neutrons in potassium is 19 and number of neutrons is 20.
What is periodic groups?Groups in periodic table are vertical columns with elements of similar physical and chemical properties. All elements are classified into different groups based in the number of valence shell electrons.
Elements of same group have same number of valence electrons. The element magnesium Mg have 12 electrons with 2 valence electrons. Its group members are shown under Mg in the column and they are calcium, strontium and rubidium.
Number of electrons in an tom is equal to the number of protons and this is called the atomic number. The atomic number o potassium is 19 and it have 19 electrons and protons.
Number of electrons = mass number-number of protons
= 39 -19 =20.
Hence, potassium (K) have 19 electrons and protons and 20 neutrons.
To find more about potassium, refer the link below:
https://brainly.com/question/13321031
#SPJ1
What forces typically hold ions together?
O A. Intermolecular forces
OB. Ionic attractions
OC. Metallic bonds
O D. Covalent bonds
Answers
Answer: Ionic attractions
Explanation:
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
Denise wants to know which time of day is the warmest. She creates a chart to record the time and temperature at various times over several days. Which of the following best describes why Denise should keep detailed notes from her experiment?
to prevent others from doing the same investigation
to prevent others from making mistakes when doing the investigation
to allow someone to practice note-taking skills when doing science investigations
to allow someone to do the same investigation and check the accuracy of results
Answers
Denise ought to keep detailed record of the experiment to allow someone to do the same investigation and check the accuracy of results. Option D
What is an experiment?The term experiment has to do with cause and effect relationships. We know that the cause is what leads to the effect if the process can be tested in a laboratory. The experiment that as performed by Denise was targeted at finding what time of the day is warmest.
From the results of the experiment, we can conclude that Denise ought to keep detailed record of the experiment to allow someone to do the same investigation and check the accuracy of results.
Learn more about experiment:https://brainly.com/question/11256472
#SPJ1
PLEASEEEEEEEEEEEEEEE I NEED THEM DONE ASAPPPPPPPPP I CANT WAIT
THE QUESTIONS ARE GONNA DETERMINE 30% OF MY GRADE SO PLEASE EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE ._.





Answers
Answer:
lol good luck
Explanation:
u need it
is carbon dioxide gas an element or a compound
Answers
Answer:
Carbon Dioxide gas is a compound.
How did the scientists know that atoms of element 115 had been created?
Answers
=》 ᴛʜᴇʏ ʟᴏᴏᴋ ꜰᴏʀ ᴛʜᴇ ᴅᴇᴄᴀʏ ᴘʀᴏᴅᴜᴄᴛꜱ. ᴛʜᴇʏ ʟᴏᴏᴋ ꜰᴏʀ ᴛᴇʟʟᴛᴀʟᴇ ꜱɪɢɴꜱ ꜰᴏʀ ᴡʜᴇɴ 115 ᴅɪꜱɪɴᴛᴇɢʀᴀᴛᴇꜱ, ʙʏ ᴡʜᴀᴛ'ꜱ ᴄᴀʟʟᴇᴅ ᴀʟᴘʜᴀ ᴘᴀʀᴛɪᴄʟᴇ ᴇᴍɪꜱꜱɪᴏɴ. ᴡʜᴇɴ ᴛʜᴇʏ ꜱᴇᴇ ᴇɴᴏᴜɢʜ ᴏꜰ ᴛʜᴏꜱᴇ ꜱɪɢɴᴀʟꜱ, ᴛʜᴇʏ ᴄᴀɴ ꜱᴀʏ ᴛʜᴇʏ ᴘʀᴏʙᴀʙʟʏ ꜰᴏʀᴍᴇᴅ ᴀ ɴᴇᴡ ᴇʟᴇᴍᴇɴᴛ.
Given 1 in=2.54 cm, how many centimeters are in an average hand (9.50 inches) __ cm (3 significant figures)
Answers
Answer:
9.5 inches = 24.1 cm
Explanation:
Given that,
1 inch = 2.54 cm
We need covert 9.5 inches to cm. For this we will use unitary method. To convert 9.5 inches to cm multiply 2.54 and 9.5. So,
9.5 inches = (2.54 × 9.5) cm
9.5 inches = 24.13 cm
or
9.5 inches = 24.1 cm
Hence, this is the required solution.
Which of the following is the best way to model the process of weathering?
Using a fan on a pile of sand causing the sand to move into a pile in a new location.
Using a ramp to dump sand at the bottom causing the sand to pile up.
Using a watering can sprinkle water onto sand causing the sand to move to a new location.
Filling a glass bottle with water and allowing it to freeze causing the bottle to break.
Answers
The best model of weathering is using a fan on a pile of sand causing the sand to move into a pile in a new location.
Weathering refers to the gradual breakdown of a rock due to the agents of denudation. The agents of denudation could be physical, chemical or biological.
The breakdown of rocks leads to the formation of soil. The closest model to the weathering of rock is using a fan on a pile of sand causing the sand to move into a pile in a new location.
The pile represents the rock which is being broken down as the fan blows the pile and it settles on a new location.
Learn more: https://brainly.com/question/14426457
the knives used in gks can: i. generate free radicals. ii. excite electrons to higher energy levels. iii. eject electrons from molecular orbitals.
Answers
The correct option is C, Knives are made from materials which includes metallic, which do now not generally generate unfastened radicals, and eject electrons from molecular orbitals.
Molecular orbitals are regions of space round a molecule wherein the possibility of finding an electron is excessive. they may be shaped by using the combination of atomic orbitals, which can be areas of space round an atom where the chance of finding an electron is high. whilst two or more atomic orbitals overlap, they integrate to shape molecular orbitals.
There are forms of molecular orbitals: bonding and antibonding. Bonding molecular orbitals result from positive interference of the atomic orbitals, whilst antibonding molecular orbitals result from damaging interference. Electrons in bonding molecular orbitals assist to maintain the atoms together in a molecule, at the same time as electrons in antibonding molecular orbitals weaken the bond.
To learn more about Molecular orbitals visit here:
brainly.com/question/30907237
#SPJ4
11. Identify the relationship between each pair of molecules. Are they identical, constitutional isomers, enantiomers, diastereomers or not related at all (not isomers)? Br O CI Cl CH2CH ICH CH OH OH онон CH2CH3 CH2CH3 Cl 12. A meso compound has no enantiomer, but can have a diastereomer. Explain and give examples. 13. Does molecule M have a diastereomer? Explain why or why not. CH,CH2 CH,CH Br H 14. For n chiral centers in a molecule, there can be 2" possible stereoisomers. 2,4-dichloropentane has 2 chiral centers, but only has 3 stereoisomers. Explain, using structures.
Answers
The question pertains to stereochemistry and involves the identification of the relationship between pairs of molecules, the concept of meso compounds and diastereomers, and the determination of the number of stereoisomers in a molecule with chiral centers.
Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules and their effects on chemical and physical properties. Identifying the relationship between pairs of molecules involves determining whether they are identical, constitutional isomers, enantiomers, diastereomers, or not isomers at all. Meso compounds are molecules with multiple chiral centers but possess an internal plane of symmetry, making them optically inactive and having no enantiomer but can have diastereomers.
The determination of whether molecule M has a diastereomer involves identifying whether it has multiple chiral centers and if there are different spatial arrangements of the substituents around them. The number of stereoisomers in a molecule with chiral centers can be determined using the formula 2^n, where n is the number of chiral centers. The explanation for why 2,4-dichloropentane has only 3 stereoisomers despite having 2 chiral centers involves the identification of meso compounds and the presence of a plane of symmetry. Understanding stereochemistry is important in many areas of chemistry, including organic chemistry, biochemistry, and materials science.
For more similar questions on topic Stereochemistry.
https://brainly.com/question/13266152
#SPJ11
Calculate the pH of a 1.4 x 10-4 M solution of HCI.

Answers
Answer:
Here is the solution....hope it helps :)

how is it d?explain please?! i do not understand.please dont guess
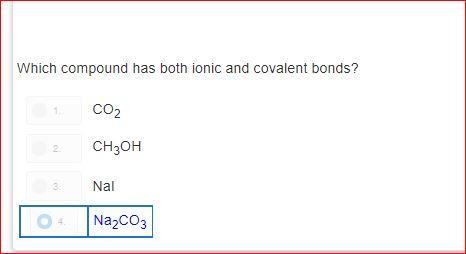
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
Among the groups of elements listed below, which have the same number of electrons in their outermost energy levels?
O NP. As, Sb
None of these
OK, Ca, Rb, Sr
OLI, B, C F
O Na Mg, ALS
Answers
Answer:
N, P, As, Sb
Explanation:
Elements present in the same group having same number of valence electrons. The first option is correct from given options because nitrogen, phosphorus, arsenic and antimony are present in same group thus having same number of electrons in outer most energy level.
Electronic configuration of N:
N₇: [He] 2s² 2p³
Electronic configuration of P:
P₁₅: [Ne] 3s² 3p³
Electronic configuration As:
As₃₃: [Ar] 3d¹⁰ 4s² 4p³
Electronic configuration Sb:
Sb₅₁: [Kr] 4d¹⁰ 5s² 5p³
Here it is clear from electronic configuration all these elements have five number of valence electrons.
What is the name for the CENTER of a spiral galaxy? What KIND of stars live in the center of a
spiral galaxy?
Answers
Which gas makes your voice deeper or has the opposite effect on you than helium when you inhale it.
Answers
it’s an inert gas that is known to be six times heavier than the air we breathe. While Helium being lighter makes our voice higher, SF6 makes our voice deeper. The sound travels slower in denser gases which is why our voice will come out deeper and rather slow.
Answer:
Sulfur hexafluoride
Explanation:
It is heavier than air that we breathe so it has an opposite effect.
Hope this helps. Pls give brainliest. Also pls sub to kgirl633 on yt!
Water near the surface of a tropical ocean has a temperature of 298.2 K(250
∘
C), whereas water 700 m beneath the surface has a temperature of 280.2 K(7.0
∘
C). It has been proposed that the warm water be used as the hot reservoir and the cool water as the cold reservoir of a heat engine. Find the maximum possible efficiency for such an engine. Analytical solution is give. Convert the same into the necessary generalized function [4]
Answers
The maximum possible efficiency for such an engine is 6.039 %.
Temperature of water near the surface of a tropical ocean = 298.2 K
Temperature of water 700 m beneath the surface = 280.2 K
To find the maximum possible efficiency for the given heat engine,
The maximum possible efficiency of a heat engine depends only on the temperatures of the hot and cold reservoirs, and is given by Carnot efficiency, η = (T₁ - T₂)/T₁
whereT₁ is the temperature of hot reservoir, T₂ is the temperature of cold reservoir. Temperature is given in Kelvin.
The temperature difference between the hot and cold reservoirs is, T₁ - T₂ = 298.2 K - 280.2 K = 18 K
Substitute these values in the Carnot efficiency equation,
Carnot efficiency, η = (T₁ - T₂)/T₁ = (18 K)/298.2 K = 0.06039. The maximum possible efficiency for such an engine is 6.039 %.
Generalised function is given as η = (T₁ - T₂)/T₁
Learn more about efficiency of Carnot engine: https://brainly.com/question/27359482
#SPJ11
CuS AICI PbOPbO Soluble Ca(C₂H₂O₂)₂ NaNO3 Answer Bank MgSO4 Mg(OH)₂ Insoluble Sr3(PO4)2 BaCO3 Ques
Answers
Among the given substances, CuS, PbOPbO, Ca(C₂H₂O₂)₂, NaNO₃, MgSO₄, and Mg(OH)₂ are soluble, while Sr₃(PO₄)₂ and BaCO₃ are insoluble.
Solubility refers to the ability of a substance to dissolve in a solvent. In this case, we are determining the solubility of the given substances.
Copper(II) sulfide (CuS) is a compound that is soluble in water. It dissociates into copper(II) ions (Cu²⁺) and sulfide ions (S²⁻) when dissolved.
Lead(II) oxide (PbOPbO) is also soluble in water. It dissociates into lead(II) ions (Pb²⁺) and oxide ions (O²⁻) when dissolved.
Calcium oxalate (Ca(C₂H₂O₂)₂) is soluble in water. It dissociates into calcium ions (Ca²⁺) and oxalate ions (C₂H₂O₂²⁻) when dissolved.
Sodium nitrate (NaNO₃) is a soluble compound. It dissociates into sodium ions (Na⁺) and nitrate ions (NO₃⁻) in water.
Magnesium sulfate (MgSO₄) is a soluble compound. It dissociates into magnesium ions (Mg²⁺) and sulfate ions (SO₄²⁻) when dissolved.
Magnesium hydroxide (Mg(OH)₂) is also soluble in water. It dissociates into magnesium ions (Mg²⁺) and hydroxide ions (OH⁻) when dissolved.
On the other hand, strontium phosphate (Sr₃(PO₄)₂) and barium carbonate (BaCO₃) are insoluble compounds. They do not readily dissolve in water and remain as solid particles when added to water.
In summary, CuS, PbOPbO, Ca(C₂H₂O₂)₂, NaNO₃, MgSO₄, and Mg(OH)₂ are soluble in water, while Sr₃(PO₄)₂ and BaCO₃ are insoluble.
Learn more about Solubility here: https://brainly.com/question/31493083
#SPJ11
Microscopic interface asymmetry and spin-splitting of electron subbands in semiconductor quantum structures. Solid State Commun
Answers
The microscopic interface asymmetry of grown semiconductor heterostructures.
The dispersion of restricted electrons. beginning from a multiband envelope formulation we practice matrix perturbation theory to derive specific expressions. Interface asymmetry, which in the conduction band Hamiltonian appear as a warping and a spin-splitting term. The warping term consequences in an inequivalence of the dispersion.
The microscopic interface asymmetry of grown semiconductor heterostructures that gives upward thrust to heavy-light hole coupling even at 0 in-plane wave vector, modifies also the dispersion of restricted electrons. beginning from a multiband envelope method we practice matrix perturbation principle to derive explicit expressions as a result of this interface asymmetry, which inside the conduction band.
Learn more about Microscopic interface here:-https://brainly.com/question/26348652
#SPJ4
definition of spirilla
don't write incorrect answer
Answers
Answer:
a bacterium with a rigid spiral structure,found in stagnant water and something causing disease
jawaban
7. Which of the following would represent the greatest pressure A) 0.680 atm B) 517 mmHg C) 11.4 psi D) 62106 Pa E) 14.1
Answers
Out of the given options, the greatest pressure would be represented by option D) 62106 Pa.
In order to compare the pressures given in different units, we can convert them to a common unit.
Here, we can convert them to the SI unit of pressure which is pascal (Pa).1 atm = 101325 Pa (approximately)1 mmHg = 133.322 Pa (approximately)1 psi = 6894.76 Pa (approximately)
So, we have:0.680 atm = 0.680 × 101325 Pa ≈ 69057.6 Pa517 mm Hg = 517 × 133.322 Pa ≈ 68910.2 Pa11.4 psi = 11.4 × 6894.76 Pa ≈ 78767.5 Pa62106 Pa = 62106 Pa14.1 = unclear (there is no unit provided)
Therefore, Out of these, the greatest pressure is represented by option D) 62106 Pa as it is already in pascals.
To know more about pressures click here.
https://brainly.com/question/30673967
#SPJ11
Part 1. A chemist reacted 15.0 liters of F2 gas with NaCl in the laboratory to form Cl2 and NaF. Use the ideal gas law equation to determine the mass of NaCl that reacted with F2 at 280. K and 1.50 atm.
F2 + 2NaCl → Cl2 + 2NaF
Part 2. Explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at STP.
Answers
Taking into account the reaction stoichiometry and ideal gas law, the mass of NaCl that reacted with F₂ at 280 K, 15 L and 1.50 atm is 114.56 grams; and if you have the same volume of fluorine gas at STP, the mass of NaCl reacted is 78.323 grams.
Reaction stoichiometryIn first place, the balanced reaction is:
F₂ + 2 NaCl → Cl₂ + 2 NaF
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
F₂: 1 moleNaCl: 2 molesCl₂: 1 moleNaF: 2 molesThe molar mass of the compounds is:
F₂: 38 g/moleNaCl: 58.45 g/moleCl₂: 70.9 g/moleNaF: 42 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
F₂: 1 mole×38 g/mole= 38 gramsNaCl: 2 moles×58.45 g/mole= 116.9 gramsCl₂: 1 mole×70.9 g/mole= 70.9 gramsNaF: 2 moles×42 g/mole= 84 gramsIdeal gas lawAn ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of gases:
P×V = n×R×T
STP conditionsThe STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
PART 1You know for F₂:
P= 1.50 atmV= 15 Ln= ?R= 0.082 (atm×L)÷(mol×K)T= 280 KReplacing in the definition of ideal gas law:
1.50 atm× 15 L = n× 0.082 (atm×L)÷(mol×K)× 280 K
Solving:
(1.50 atm× 15 L)÷ (0.082 (atm×L)÷(mol×K)× 280 K)= n
0.979965 moles= n
Then the following rule of three can be applied: if by stoichiometry of the reaction 1 mole of F₂ reacts with 116.9 grams of NaCl, 0.979965 moles of F₂ reacts with how much mass of NaCl?
mass of NaCl= (0.979965 moles of F₂× 116.9 grams of NaCl)÷ 1 mole of F₂
mass of NaCl= 114.56 grams
Finally, the mass of NaCl reacted is 114.56 grams.
PART 2In this case, you have the same volume of fluorine gas at STP:
P= 1 atmV= 15 Ln= ?R= 0.082 (atm×L)÷(mol×K)T= 273 KReplacing in the definition of ideal gas law:
1 atm× 15 L = n× 0.082 (atm×L)÷(mol×K)× 273 K
Solving:
(1 atm× 15 L)÷ (0.082 (atm×L)÷(mol×K)× 273 K)= n
0.67 moles= n
Then the following rule of three can be applied: if by stoichiometry of the reaction 1 mole of F₂ reacts with 116.9 grams of NaCl, 0.67 moles of F₂ reacts with how much mass of NaCl?
mass of NaCl= (0.67 moles of F₂× 116.9 grams of NaCl)÷ 1 mole of F₂
mass of NaCl= 78.323 grams
Finally, the mass of NaCl reacted is 78.323 grams.
Learn more about reaction stoichiometry, ideal gas law and STP conditions:
brainly.com/question/27809250
brainly.com/question/28096494
brainly.com/question/27815860
#SPJ1
If there are 2 moles of AI(NO3)3, then there are ___ moles of aluminum, ___ moles kf nitrogen, and ___ moles of oxygen.
Answers
If there are 2 moles of Al(NO3)3, then there are 2 moles of aluminum (Al), 6 moles of nitrogen (N), and 18 moles of oxygen (O).
The compound Al(NO3)3 contains one aluminum atom (Al), three nitrate ions (NO3-), and a total of nine oxygen atoms (three oxygen atoms per nitrate ion). The subscript 3 in Al(NO3)3 indicates that there are three nitrate ions in one formula unit of the compound.
To determine the number of moles of each element, we multiply the number of moles of the compound (2 moles) by the corresponding coefficient for each element from the compound's formula. In this case, we have:
- 2 moles of Al(NO3)3 * 1 mole of Al per mole of Al(NO3)3 = 2 moles of Al
- 2 moles of Al(NO3)3 * 3 moles of N per mole of Al(NO3)3 = 6 moles of N
- 2 moles of Al(NO3)3 * 9 moles of O per mole of Al(NO3)3 = 18 moles of O
Therefore, if there are 2 moles of Al(NO3)3, there are 2 moles of aluminum, 6 moles of nitrogen, and 18 moles of oxygen.
To learn more about aluminum click here: brainly.com/question/28989771
#SPJ11
JWhat type of evidence is gathering information through your senses or using scientific tools?
Predictions
Results
Scientific research
Observations
Answers
Blasting caps containing Lead Azide detonate. How fast is the chemical reaction occurring? Subsonic speeds (slower than the speed of sound) Supersonic speeds (faster than the speed of sound) Submit Activate Win
Answers
The chemical reaction in blasting caps containing Lead Azide occurs at supersonic speeds, indicating a highly explosive and rapid reaction. The release of energy is sudden and violent, leading to the detonation of the blasting caps.
The detonation of blasting caps containing Lead Azide can occur at both subsonic and supersonic speeds, depending on the conditions and the specific characteristics of the reaction.
At subsonic speeds, the chemical reaction occurs relatively slowly compared to the speed of sound. The reaction proceeds through a series of chemical reactions and propagation of shockwaves within the blasting cap. The reaction front moves at speeds lower than the speed of sound, resulting in a relatively slower detonation process.
On the other hand, at supersonic speeds, the chemical reaction occurs rapidly, faster than the speed of sound. The detonation process involves a high-speed shockwave that propagates through the blasting cap, causing almost instantaneous chemical reactions and energy release. This supersonic detonation can result in a more rapid and violent explosion.
The specific speed at which the chemical reaction occurs in Lead Azide blasting caps depends on various factors such as the initiation mechanism, the confinement conditions, and the specific composition of the explosive material.
Learn more about Lead Azide here:
https://brainly.com/question/29622816
#SPJ4
What is the chemical formula for sodium sulfate
Answers
Answer:
Na2So4
Explanation:
Na is group 1, so has a charge of 1+
Sulphate’s formula is SO4 2-,
because sulphate has double the charge of Na, you need two Na’s to make it equal.
Therefore it’s Na2SO4
When two additional electrons are required to follow the octet rule a ____________ bond is created
Answers
Answer
o double covalent bond is cteated.
why does a freely suspended iron rod does not point at N-S directions always
Answers
Answer:
This is because their is a repulsive force between the magnetic north and the geographical north since the rod experiences the Earth's magnetic field.
The iron rod deflects at an angle (angle of dip) from the horizontal.