Is this atom more likely to gain electrons or to lose electrons? Explain how you can tell
e
e-
e е
es
e е
16P
16 N
e es
Please help

Answers
Answer:
Likely to gain electrons
Explanation:
The atom shown is likely to gain additional electrons to complete its electronic configuration.
Since this is a neutral specie, the number of protons and electrons are the same. The atom has 16 electrons the number of valence electrons is 6 If the atom gains two additional electrons, the octet configuration is attainedAlso, the atom can lose 6 electrons to become an octetThe atom will prefer to gain additional 2 electrons to give an octet configuration.
Related Questions
why is carbon monoxide used to reduce zinc oxide to zinc in the zinc extraction process and not carbon directly?
Answers
Answer:
The chemical reaction involving the reduction of ZnO by CO is not feasible thermodynamically: This is because the standard free energy of formation ( ) of CO2 from CO is higher than that of the formation of ZnO from Zn. Thus, CO cannot be used to reduce ZnO to Zn.
Find the density of a substance that’s had a volume of 24.7mL and a mass of 49.1g. Give the answer with two decimals.
Answers
Answer:
1.99 g/mL
Explanation:
Use the formula d = m/v, where m is the mass and v is the volume.
Plug in the values:
d = 49.1/24.7
d = 1.99 g/mL
Answer:
About 1.99 g/mL
Explanation:
The density of a substance can be found using the following formula:
d= m/v
where m is the mass and v is the volume.
The mass of this substance is 49.1 grams and the volume is 24.7 milliliters.
m= 49.1 g
v= 24.7 mL
Substitute these values into the formula.
d= 49.1 g / 24.7 mL
Divide 49.1 g by 24.7 mL
d= 1.98785425 g/mL
We are asked to round to two decimal places, or the hundredth place. The 8 in the thousandth place tells us to round the 8 to a 9 in the hundredth place.
d ≈ 1.99 g/mL
The density of the this substance is approximately 1.99 grams per milliliter.
Lipase is an enzyme that breaks down the fat in food molecules into smaller molecules. Which of the following best describes the function of lipase?
Answers
Explain why NOBLE gases do not for ions
Answers
Answer/Explanation:
They have very high ionization energies so they don't form ions. The noble gases are the least reactive of all the elements but the heavier ones do form some molecules. Helium and neon never form molecules. They have completely filled electron shells with no have-filled orbitals available for making covalent bonds
After the krebs cycle only a small portion of the energy of glucose has been converted to atp. At this point the majority of usable energy is contained in.
Answers
At this point, most of the available energy is contained in the reduced electron carriers NADH and FADH₂.
What is oxidative phosphorylation?Oxidative phosphorylation or electron transfer-associated phosphorylation or terminal oxidation is a metabolic pathway in which cells use enzymes to oxidize nutrients and release chemical energy to produce adenosine triphosphate. This takes place in mitochondria in eukaryotes.
Oxidative phosphorylation often produces the greatest amount of energy produced within the cell. The combination of glycolysis and the citric acid cycle of the Kreb cycle alone yields only four ATPs. Most or most of the remaining energy is stored in the electron carriers NADH and FADH₂.
To know more about oxidative phosphorylation visit:
https://brainly.com/question/29104155
#SPJ4
what force was applied to an object if 35 j of work was done and the object moved 7 m?
Answers
5N force was applied to an object if 35 j of work was done and the object moved 7 m
A force is an effect that can alter an object motion according to physics and an object with mass can change its velocity or accelerate as a result force and also force is as push or pull and work done by the force on an object means assuming that direction of the force is parallel to the displacement of the object
Then the formula is
W = f × d
Where f is the magnitude of force and d is the displacement of the object
Then W = 35 J is the work done and
d = 7m is the displacement of the object
So rearrange the equation then
F = 35/7 = 5N
5N force was applied to an object if 35 j of work was done and the object moved 7 m
Know more about object
https://brainly.com/question/11748734
#SPJ9
what method will you use to separate the mixture of two solids which have different solubility in water
Answers
Answer:
A fractional crystallisation method is used for separating a mixture of two solids, if their solubilities in a particular solvent differ widely.
Explanation:
Calculate the percent ionic, the percent covalent, and the bond length (in picometers) of a chemical bond between phosphorus and selenium.
Phosphorus—atomic radius: 109 pm; covalent radius: 106 pm; ionic radius: 212 pm.
Selenium—atomic radius: 122 pm; covalent radius: 116 pm; ionic radius: 198 pm.
98 percent ionic, 2 percent covalent, 410 pm
4 percent ionic, 96 percent covalent, 222 pm
2 percent ionic, 98 percent covalent, 222 pm
96 percent ionic, 4 percent covalent, 410 pm
Answers
Answer:
The correct option is;
4 percent ionic, 96 percent covalent, 222 pm
Explanation:
The parameters given are;
Phosphorus:
Atomic radius = 109 pm
Covalent radius = 106 pm
Ionic radius = 212 pm
Electronegativity of phosphorus = 2.19
Selenium:
Atomic radius = 122 pm
Covalent radius = 116 pm
Ionic radius = 198 pm
Electronegativity of selenium= 2.55
The percentage ionic character of the chemical bond between phosphorus and selenium is given by the relation;
Using Pauling's alternative electronegativity difference method, we have;
\(\% \, Ionic \ Character = \left [18\times (\bigtriangleup E.N.)^{1.4} \right ] \%\)
Where:
Δ E.N. = Change in electronegativity = 2.55 - 2.19 = 0.36
Therefore;
\(\% \, Ionic \ Character = \left [18\times (0.36)^{1.4} \right ] \% = 4.3 \%\)
Hence the percentage ionic character = 4.3% ≈ 4%
the percentage covalent character = (100 - 4.3)% = 95.7% ≈ 96%
The bond length for the covalent bond is found adding the covalent radii of both atoms as follows;
The bond length for the covalent bond = 106 pm + 116 pm = 222 pm.
The correct option is therefore, 4 percent ionic, 96 percent covalent, 222 pm.
What is the smallest representative particle of an element?
Answers
Answer:
atom
Explanation:
The smallest representative particle of an element is an atom.
What are atomsAtoms are the fundamental building blocks of matter and the smallest unit of an element that retains the chemical properties of that element. Each atom consists of a nucleus at the center, composed of positively charged protons and neutral neutrons, surrounded by a cloud of negatively charged electrons.
Atoms combine to form molecules or ions through chemical reactions, but they themselves represent the basic structure and identity of an element. Each element has a unique number of protons in its nucleus, known as its atomic number, which determines its position on the periodic table and its distinct chemical properties.
Learn more about element at
https://brainly.com/question/20096027
#SPJ6
What is the density of a block with a mass of 36 g and a volume of 9 cm3?
Answers
Answer:
The answer is
4.0 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \)From the question
mass of block = 36 g
volume = 9 cm³
The density is
\(density = \frac{36}{9} \)
We have the final answer as
4.0 g/cm³
Hope this helps you
In the image below, the truck and bus have the same mass and are
traveling at the same speed. Which of these statements accurately
explains their energies? *
A) They have the same gravitational potential energy but have different kinetic
energies
B) They have the same kinetic energy but different gravitational potential energies.
C) The truck has more kinetic energy than the bus.
D) The truck has more gravitational potential energy than the bus

Answers
Answer:
B)They have the same kinetic energy but different gravitational potential energies.
Explanation:
I don't know the exact explanation but I just took the test.
What is the total amount of electrons for Mg2-
Answers
Answer:
10 electrons
There are 10 electrons in a Mg2+ ion
Explanation:
typical activation energies, ea, of many reactions are either less than or about 100 kj/mol. if cole calculated an ea of 1200 kj/mol for the reaction of kmno4 with malonic acid, then his calculated value can be considered reasonable.truefalse
Answers
Answer:
False
Explanation:
The problem states that typical activation energies are ≤ 100 kJ/mole.
The KMnO4 reaction with malonic acid is said to have an activation energy of 1200 kJ.mole.
We aren;t given the know activation energy, but are asked to form a judgement based a statement that typical activation energies are generally less than or equal to 100 kJ/mole.
With no other information, we should consider his calculated value to be unreasonable. So the answer is FALSE. His calculated value cannot be considered reasonable.
How many moles are in 85.62 g of K,C03?
Answers
In the example pictured, the soda bottle would act as the . the kinetic energy of the soda bottle will be converted into the of the beanbag. the higher the beanbag rises, the greater the .
Answers
The soda bottle acts as the initial source of energy, transferring its kinetic energy to the beanbag, which then gains potential energy as it rises. The higher the beanbag rises, the greater the amount of energy transferred from the soda bottle.
In the example pictured, the soda bottle would act as the initial source of energy. The soda bottle, when released, possesses potential energy due to its elevated position. As it falls, this potential energy is converted into kinetic energy, which is the energy of motion. The soda bottle's kinetic energy is transferred to the beanbag upon impact, causing it to move.
The kinetic energy of the soda bottle will be converted into the potential energy of the beanbag. When the soda bottle collides with the beanbag, the energy is transferred, causing the beanbag to rise. As the beanbag rises higher, its potential energy increases, while the kinetic energy of the soda bottle decreases.
The relationship between the height of the beanbag and the energy transferred can be explained through the conservation of energy. According to the law of conservation of energy, energy cannot be created or destroyed but can only be transferred or transformed from one form to another. Therefore, as the beanbag rises higher, it gains more potential energy, indicating that a greater amount of energy has been transferred from the soda bottle.
Learn more about potential energy
https://brainly.com/question/21175118
#SPJ11
a 100 ml solution contains 0.100 m formic acid (pka 3.75) and 0.100 m sodium formate. if 1.00 ml of 0.050 m hcl were added, what would be the ph of the solution?
Answers
Answer:
ngl ts ngl ts ts ngl...imma keep it a buck.96 ion got the answers kanye do.
Explanation:
Identifying Variables in the Effect of Type of Material on
Absorption Experiment
Answer the questions using the drop-down menus.
Which is the independent variable in this experiment?
Which is the dependent variable in this experiment?
Which is a controlled variable in this experiment?
Answers
In Absorption Experiment,
Water location is the independent variable in this experiment.
Room Temperature in the material is dependent variable in this experiment.
Type of a material is a controlled variable in this experiment.
What is Absorption Experiment ?Absorption experiment is nothing but the experiment of absorption of water in a material (sponge).
Experiment requires vessel, water and sponge.
Processor - take a vessel, pour water into it and dip sponge. take out the sponge. After taking of out sponge, water appears to be decreased from the vessel and sponge gets heavier. When we squeeze the the sponge, water drops down. It is because of water is absorbed by the spouge.
There are number of pores in the sponge, which absorbs the water.
Water location is the independent variable.
In this experiment Room Temperature is dependent variable in this experiment rate of absorption increases with increase in the temperature. decrease in the temperature cause to solidify the water. Type of a material is a controlled variable, we can change type of the material.
To know more about variables :
brainly.com/question/14289293
#SPJ7.
2). In the reaction sequence (parallel) shown below, A⟶k0D,A⟶k11U a) Calculate the selectivity. b) Explain how you increase selectivity by manipulating species A concentration (CA). Justify
Answers
a) The selectivity of a reaction is defined as the ratio of the rate of formation of the desired product to the sum of the rates of formation of all products. In this case, we have two parallel reactions: A ⟶ D and A ⟶ U. The selectivity (S) can be calculated as:
S = (rate of formation of D) / (rate of formation of D + rate of formation of U)
b) Manipulating the concentration of species A (CA) can affect the selectivity by altering the reaction rates of the individual pathways. By increasing or decreasing the concentration of A, we can favor one reaction pathway over the other, thereby increasing selectivity.
To justify this, let's consider the rate equations for the two reactions:
Rate of formation of D = k0 * CA
Rate of formation of U = k11 * CA
From the rate equations, it is clear that the rates of formation for both D and U are directly proportional to the concentration of A (CA). By increasing CA, both reaction rates will increase. However, the relative increase in the rate of the desired product (D) will be larger compared to the increase in the rate of the undesired product (U) if the rate constants (k0 and k11) are significantly different.
Thus, by manipulating the concentration of A, we can adjust the ratio of the reaction rates and bias the system towards the desired product, thereby increasing selectivity.
To know more about formation click this link -
brainly.com/question/17030902
#SPJ11
Compound 1 was designed to exhibit pH-dependent self-assembly. What feature(s) of the molecule is(are) responsible for the pH dependence of aggregation (Equation 1)?
Answers
The feature(s) of Compound 1 that are responsible for the pH dependence of aggregation in Equation 1 are likely the presence of acidic or basic functional groups within the molecule.
Depending on the pH of the environment, these functional groups may be protonated or deprotonated, leading to changes in the overall charge and structure of the molecule. These changes can then affect the interactions between multiple molecules of Compound 1, leading to differences in self-assembly and aggregation behavior at different pH values.
Compound 1's pH-dependent self-assembly can be attributed to the presence of ionizable functional groups in the molecule. These groups, such as carboxylic acids or amines, undergo protonation or deprotonation depending on the pH, which in turn influences the molecule's aggregation behavior (Equation 1). This change in aggregation is due to alterations in the molecule's charge, solubility, and intermolecular interactions as the pH changes.
Visit here to learn more about functional groups brainly.com/question/14618322
#SPJ11
Cutting, melting, bending, or crushing are examples of what kind of change?
Answers
Answer:
those are all physical changes and most physical changes can be undone like crushing a pice of metal you can flatten it back out you anwser is physical change
Explanation:
Answer:
It is a physical change because it can be reversed back and no new subtances is dormed.
What keeps a satellite in orbit around Earth?
A.
Earth’s gravity
B.
Earth’s atmosphere
C.
Earth’s solar system
D.
Earth’s magnetic field
Answers
Answer:
I think its A. Earths gravity
Explanation:
Hope this answer helps!
PLEASE HELP
This tree species was preserved as a fossil in the Arizona desert. The species is now extinct. Describe what the environment was like when the tree lived and how it is different now. Then, infer why this type of tree and other living things that lived with it are extinct
Answers
When the tree species was preserved as a fossil in the Arizona desert, the environment was likely different compared to the present. Based on the fact that the tree species is now extinct, infer that the conditions that supported its existence have significantly changed.
What leads to extinction?In the past, the environment where the tree lived may have had different climatic conditions, such as temperature, precipitation patterns, and humidity levels. It may have been part of a specific ecosystem or habitat that provided suitable resources for the tree's growth and survival. The tree species may have had adaptations that allowed it to thrive in that particular environment.
The extinction of the tree species may have had cascading effects on other living things that were associated with it. For example, other plant species that relied on the tree for shade or as a host for epiphytic growth might have also suffered or disappeared. Similarly, animals that depended on the tree for food, shelter, or nesting sites could have been affected by its extinction.
Find out more on extinction here: https://brainly.com/question/1027555
#SPJ1
solve for x 5 to the power 2 x + 4 - 25 to the power x - 1 is equals to
Answers
Answer:
[][][][][][][][][][][][][][]
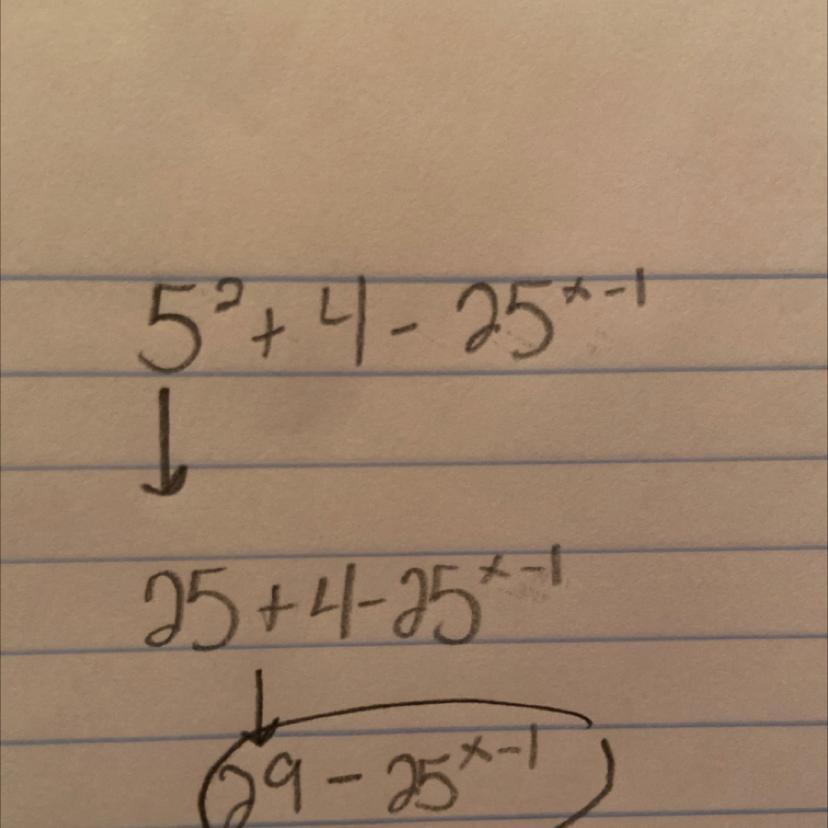
Which of the following locations is the best climate to promote chemical weathering? 1. Death Valley 2. Dominican Republic rain forest 3. Siberia 4. Alaskan tundra 5. Sahara desert
Answers
The Dominican Republic rain forest is the location that has the best climate to promote chemical weathering.
Chemical weathering occurs when the minerals that compose rocks are altered through chemical reactions that happen when they come into contact with water and air. This process happens much faster in locations where the climate is hot and wet. This is because the combination of heat and water can break down the minerals in rocks more quickly than in colder, drier locations.
Locations such as the Dominican Republic rainforest, which are characterized by high temperatures and high humidity, are ideal for chemical weathering. This is because the combination of heat and moisture accelerates chemical reactions, which break down minerals in rocks. Hence, the Dominican Republic rainforest is the location that has the best climate to promote chemical weathering.
Learn more about weathering from the given link
https://brainly.com/question/829782
#SPJ11
Drag each tile to the correct image.
Match each alkane name with its structure.
octane
decane
propane
butane
heptane
CHE
IGH
CHE
Reset
Next

Answers
Answer:
The first one is Propane
The second one is HEPTANE
The third one is octane
The 4th is butane
the 5th is decane
The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
The images has been the representation of the ball and stick structure of the compounds. The central balls have been the representation of the carbon atom , with small balls attached to the sticks have been the representation of the hydrogen attached.
The following structures has been given as:
The structure has 3 carbon atoms with the presence of 8 hydrogen. The molecular formula has been \(\rm C_3H_8\). It has been the structure of propane.The structure has 7 carbon and 16 hydrogen. The structure has been the representation of heptane with molecular formula \(\rm C_7H_{16}\).The structure has molecular formula \(\rm C_8H_{18}\) with 8 carbon and 18 hydrogen. It has been named Octane, according to IUPAC.The structure with 4 carbon and 10 hydrogen with molecular formula \(\rm C_4H_{10}\) has been named according to IUPAC as butane.The structure with molecular formula \(\rm C_{10}H_{22}\) has presence of 10 carbon and 22 hydrogen. It has been named as Decane.The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
For more information about structure of hydrocarbons, refer to the link:
https://brainly.com/question/8049265
Calculate the molality of an aqueous solution that is 6. 2% by mass calcium chloride. You might need to know that the density is 1. 18 g/ml.
Answers
The molality of an aqueous solution that is 6.2% by mass calcium chloride with 1.18 g/mL density is 0.0528 mol/kg.
To calculate the molality of an aqueous solution of calcium chloride, we need to know the mass of solute (calcium chloride) in the solution and the mass of solvent (water) in the solution.
First, let's convert the concentration from percentage by mass to grams of solute per kilogram of solvent:
6.2% = 6.2 g/100 g = 0.062 g/g = 0.062 g/1000 g = 0.062 g/kg
Next, we need to determine the mass of water in the solution. We can use the density of the solution to do this. Assuming the volume of the solution is 1 mL, we have:
mass of water = volume * density = 1 mL * 1.18 g/mL = 1.18 g
Finally, we can use the mass of solute and mass of solvent to calculate the molality:
molality = moles of solute / kilograms of solvent = 0.062 g / 1.18 g = 0.0528 mol/kg (rounded to four decimal places)
Learn more about molality here: https://brainly.com/question/26921570
#SPJ4
which of the following options correctly describe the general reactions of esters? select all that apply. multiple select question. esters react readily with socl2 to produce acid chlorides. esters can be hydrolyzed either under acidic or basic conditions. an ester can react with any type of amine to produce an amide. an ester can react with ammonia to produce an amide. esters can only react with nucleophiles if the carbonyl oxygen atom is protonated.
Answers
Esters can also react with nucleophiles even if the carbonyl oxygen atom is not protonated. Therefore, the option "esters can only react with nucleophiles if the carbonyl oxygen atom is protonated" is not correct.
The correct options that describe the general reactions of esters are:
- Esters can be hydrolyzed either under acidic or basic conditions.
- An ester can react with any type of amine to produce an amide.
- An ester can react with ammonia to produce an amide.
Esters can be hydrolyzed either under acidic or basic conditions, and they can react with ammonia to produce an amide. These general reactions involve esters interacting with nucleophiles, and these reactions typically proceed more readily when the carbonyl oxygen atom is protonated.
Learn more about nucleophiles here:
brainly.com/question/31425447
#SPJ11
has 2 valence electrons. It has electron shells and is reactive than Boron. This element has the same number of electron shells as has 4 valence electrons. It has electron shells and is reactive than Aluminum. This element has the same number of electron shells as
Answers
Answer:
Beryllium.
Silicon.
Explanation:
Beryllium has 2 electrons in their outermost shell and has highly reactive than boron because Boron needs 3 electrons to lose whereas, beryllium needs to lose only 2 electrons to get stability. Silicon has 4 electrons in its outermost shell and is less reactive than aluminum because aluminum has to lose 3 electrons to get stability as compared to silicon which needs 4 electrons to lose to be stable and non-reactive.
Read the excerpt from A History of Women’s Suffrage by Stanton, Anthony, and Gage.
Another writer asserts that the tyranny of man over woman has its roots, after all, in his nobler feelings; his love, his chivalry, and his desire to protect woman in the barbarous periods of pillage, lust, and war. But wherever the roots may be traced, the results at this hour are equally disastrous to woman. Her best interests and happiness do not seem to have been consulted in the arrangements made for her protection. She has been bought and sold, caressed and crucified at the will and pleasure of her master. But if a chivalrous desire to protect woman has always been the mainspring of man's dominion over her, it should have prompted him to place in her hands the same weapons of defense he has found to be most effective against wrong and oppression.
Which statement paraphrases the authors’ argument?
Throughout history, men have strived to protect women through chivalrous action and guardianship.
Women have always been subservient to men because they have needed protection during war and pillage.
Men have not been successful protecting women because they have not given women the power to protect themselves.
During times of war, men and women rely on one another for defense, and they each have weapons for battle.
Answers
Answer:
C
Explanation:
Took the test
In her book A Vindication of the Rights of Women, British author Mary Wollstonecraft advocated that women should be treated with the same respect and decency as men, particularly when it comes to education. Thus, option C is correct.
What Women’s Suffrage by Stanton, Anthony, and Gage?A Vindication of the Rights of Woman (1792), a groundbreaking feminist text, asserts that girls should be given the same privileges as males.
So that they might become outstanding husbands, mothers, and laborers in addition to being educated to be frivolous and inept by the educational system.
Women should be given the same opportunities as men, and women share the same capacity for reasoning at birth. Women should have the same rights to influence, expertise, and power in society as men do.
Therefore, Men have failed in their attempts to safeguard women because they have not given them the means to do so.
Learn more about Women’s here:
https://brainly.com/question/2975708
#SPJ6
After 3. 00 g of solid PH3BCl3 is added to a closed 1. 500- L vessel at 60 ∘C , the vessel is charged with 0. 0500 g of BCl3(g). What is the equilibrium concentration of PH3 ?
Answers
The equilibrium concentration of PH3 cannot be determined without the equilibrium constant and reaction equation for the reaction involving PH3BCl3 and BCl3.
To determine the equilibrium concentration of PH3, we need the equilibrium constant and the balanced reaction equation for the reaction involving PH3BCl3 and BCl3. Without this information, it is not possible to calculate the equilibrium concentration of PH3. The equilibrium concentration depends on various factors such as the stoichiometry of the reaction and the equilibrium constant, which indicates the extent of the reaction at equilibrium. Without these details, we cannot accurately determine the equilibrium concentration of PH3.
Learn more about equilibrium concentration of PH3 here:
https://brainly.com/question/32555075
#SPJ11