Iodine-131 is one of the most important isotopes used in the diagnosis of thyroid cancer. One atom has a mass of 130.906114 amu. Calculate the binding energy(a) per nucleon in MeV;
Answers
Iodine-131 is one of the most important isotopes used in the diagnosis of thyroid cancer. One atom has a mass of 130.906114 amu.\
What is thyroid cancer?Cancer that originates in the tissues of the thyroid gland is known as thyroid cancer. It is a condition where cells develop improperly and are susceptible to spreading to different bodily regions. A bump in the neck or swelling are examples of symptoms. Thyroid cancer is not always diagnosed because it can move from other parts of the body to the thyroid.
Young age radiation exposure, having an enlarged thyroid, and family history are risk factors. Papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer are the four primary kinds. Ultrasound and tiny needle aspiration are frequently used in diagnosis. As of right now, it is not advised to screen those who are healthy and at normal risk for the disease.
To learn more about thyroid cancer from the given link:
brainly.com/question/11880360
#SPJ4
Related Questions
According to the kinetic molecular theory, what is different about a sample of xenon gas at 25 deg * C and another sample at 100 deg * C
Answers
The average kinetic energy of molecules in a sample of xenon gas at 100°C would be higher in comparison to the 25°C sample of xenon gas, in accordance with the kinetic molecular theory.
According to the kinetic molecular theory, a sample of xenon gas at 25°C and another sample at 100°C would have different average kinetic energies of their molecules.
At a higher temperature, the average kinetic energy, of the gas molecules increases, resulting in higher molecular speeds and more frequent collisions with the container walls. The pressure and volume of the gas sample rise as a result.
To know more about Kinetic molecular theory, visit,
https://brainly.com/question/3924326
#SPJ1
Name the following parts:
A—-
B——
C——

Answers
B—-Neutron
C—-Proton
HELP ASAP.
Determine the length of time it would take for a banana slug to run a complete marathon.
Use the information below for guidance.
Question: How long would it take a slug to run a marathon, 26.2 miles?
Provide your answer in a time length that gives a value between 1 and 100. You'll need to decide whether that is minutes, days, years, or decades. The number needs to have a context (or have meaning) to you.
Here are some potentially useful conversion factors. There are multiple ways to solve this problem and you may not need all these conversion factors. You might also need some of your metric prefix conversions! Remember "King Henry died by drinking chunky milk". ●
Length 12 inch = 1 foot
1 inch = 2.54 cm
3 feet = 1 yard
5280 feet 1 mile
1760 yards = 1 mile
Time 1 min = 60 seconds
60 minutes = 1 hour
24 hours = 1 day
365.25 days = 1 year
10 years = 1 decade
Slug Travel Rate 0.0229 mm/s
Hints:
Consider starting with the length of the marathon, 26.2 miles.
Show all your work. Include both number and unit in each space.
Use the "fence posts" to organize your conversion factors to cancel units.
Answers
It would take a banana slug approximately 1,029,863 minutes to run a complete marathon, which is equivalent to about 17,164 hours or approximately 714 days.
How to determine length of time?To determine the length of time it would take for a banana slug to run a complete marathon of 26.2 miles, use the given information and conversion factors:
Given:
Slug Travel Rate: 0.0229 mm/s
Conversion Factors:
1 mile = 5280 feet
1 foot = 12 inches
1 inch = 2.54 cm
1 cm = 10 mm
1 second = 1 s
Now set up the conversions to cancel out the units and find the time:
26.2 miles × (5280 feet/1 mile) × (12 inches/1 foot) × (2.54 cm/1 inch) × (10 mm/1 cm) × (1 s/0.0229 mm) = X seconds
Calculating this value:
X = (26.2 × 5280 × 12 × 2.54 × 10) / (0.0229)
X ≈ 61,791,789.68 seconds
Now convert seconds to a time unit that has a value between 1 and 100. Let's choose minutes:
61,791,789.68 seconds × (1 min / 60 seconds) = Y minutes
Y ≈ 1,029,863.16 minutes
So, it would take a banana slug approximately 1,029,863 minutes to run a complete marathon, which is equivalent to about 1,029,863/60 ≈ 17,164 hours or approximately 714 days.
Find out more on conversion factors here: https://brainly.com/question/97386
#SPJ1
Calculation of milliequivalents in a solution takes into account which of the 3 following factors?
concentration
atomic weight
electrical charge
Answers
Calculation of milliequivalents in a solution takes into account the concentration and electrical charge of the solute, but not the atomic weight.
The calculation of milliequivalents in a solution takes into account the following three factors: concentration, atomic weight, and electrical charge.
1. Concentration: This is the amount of solute present in a given volume of solution, typically expressed as moles per liter (mol/L). The concentration of the solute directly impacts the number of milliequivalents present in the solution.
2. Atomic weight: The atomic weight is the mass of an element in atomic mass units (amu) and is used to determine the number of moles of a substance. When calculating milliequivalents, we must consider the atomic weight in order to convert the amount of solute from mass to moles.
3. Electrical charge: Milliequivalents are a measure of the chemical combining capacity of a solute based on its electrical charge. Therefore, we must consider the electrical charge of the solute ions to determine the number of milliequivalents present in the solution.
In summary, to calculate the milliequivalents of a solute in a solution, you need to take into account the concentration of the solute, its atomic weight, and its electrical charge.
To know more about milliequivalents Visit:
https://brainly.com/question/31920642
#SPJ11
What happens to gas solubility of oxygen in the blood of a person who is placed in a hyperbaric chamber?
Answers
The solubility of oxygen in the blood of a person who is placed in a hyperbaric chamber increases.
This is because the pressure inside the hyperbaric chamber is increased, which results in an increased partial pressure of oxygen. The solubility of a gas in a liquid is directly proportional to the partial pressure of that gas, so as the pressure of oxygen increases, the solubility of oxygen in the blood increases.
As a result, more oxygen can dissolve in the blood, providing more oxygen to the tissues and organs of the body. This increased oxygen supply can help to improve oxygenation in tissues that may be deprived of oxygen, such as in cases of decompression sickness, carbon monoxide poisoning, and some wounds.
It is important to note that while the increased solubility of oxygen in the blood can provide benefits in certain cases, it can also lead to toxicity if the pressure is increased too much. Therefore, it is important to carefully monitor the pressure inside the hyperbaric chamber and adjust it as necessary to avoid any harmful effects.
Learn more about hyperbaric chamber:
brainly.com/question/28044756
#SPJ4
what products would you obtain from reaction of 1-pentanol with the following reagents? (a) pbr3 (b) socl2
Answers
(a) When 1-pentanol is reacted with PBr3 (phosphorus tribromide), it undergoes a substitution reaction known as the Appel reaction.
The reaction proceeds as follows:
1-pentanol + PBr3 → pentyl bromide + HBr + POBr3
The product of the reaction is pentyl bromide (1-bromopentane), hydrogen bromide, and phosphorus oxybromide.
(b) When 1-pentanol is reacted with SOCl2 (thionyl chloride), it undergoes an elimination reaction known as the Dehydration reaction. The reaction proceeds as follows:
1-pentanol + SOCl2 → 1-chloropentane + SO2 + HCl
The product of the reaction is 1-chloropentane, sulfur dioxide, and hydrogen chloride. This reaction involves the removal of a molecule of water from the 1-pentanol to form a carbon-carbon double bond, and the replacement of the hydroxyl group (-OH) with a chlorine atom (-Cl).
Learn more about 1-pentanol here:
https://brainly.com/question/13128647
#SPJ11
A certain atom has a nucleus containing six protons and eight neutrons and has six electrons orbiting the nucleus. This atom is a form of the element — ¡silicon ¡carbon ¡magnesium ¡calcium
Answers
Answer:
Carbon
Explanation:
The way to identify an element is by looking at the number of protons the atom has. Every element has its own atomic number. This atom has 6 protons. If you look at the periodic table, you can see that the atom with the atomic number of 6 is carbon.
As the atom has six protons in it's nucleus, the atom is a form of element of carbon.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
how can you blance it and make it equal on both sides
2H2+o2=2H2o blance it
Answers
Answer:
it have been already balanced
2H2 + O2 = 2H2O.
How many atoms of nitrogen are in 1.20 grams of aspartame?
Answers
There are approximately 4.92 x 10^21 nitrogen atoms in 1.20 grams of aspartame.
What ingredients are in aspartame?The two naturally occurring amino acids phenylalanine and aspartic acid, which are also parts of proteins in our bodies and food, are what makeup aspartame. Aspartame's sweet flavor comes from a small modification of the phenylalanine by the addition of a methyl group.
The molecular formula of aspartame is C14H18N2O5.
The molar mass of aspartame,
(14 x 12.01 g/mol) + (18 x 1.01 g/mol) + (2 x 14.01 g/mol) + (5 x 16.00 g/mol) = 294.30 g/mol
Aspartame's molecular weight in 1.20 grams can be computed as follows:
1.20 g / 294.30 g/mol = 0.00408 mol
Every aspartame molecule has two nitrogen atoms.
0.00408 mol x 2 = 0.00816 moles of nitrogen
So, we will use Avogadro's number in order to transform nitrogen moles to nitrogen atoms:
0.00816 mol x 6.022 x 10^23 atoms/mol = 4.92 x 10^21 atoms of nitrogen
To know more about the aspartame visit:
https://brainly.com/question/31299610
#SPJ9
which of the following involve digital quantities? the speedometer in a mint '67 camaro a linear thermostat a ten-position switch lightning
Answers
Out of the options provided, the following involve digital quantities: a ten-position switch.
A ten-position switch typically refers to a switch with ten different positions or settings, each representing a discrete digital value. The switch can be used to select one of the ten available options, which are usually represented by binary digits (0 or 1) or other digital codes.
On the other hand, the speedometer in a mint '67 Camaro and a linear thermostat do not involve digital quantities.
The speedometer in a '67 Camaro is a mechanical instrument that measures and displays the speed of the vehicle. It operates using a mechanical linkage connected to the vehicle's transmission or wheels. The speed is indicated by the position of a needle on an analog scale, rather than being represented digitally.
A linear thermostat is also an analog device used to control the temperature in a room or building. It typically consists of a bimetallic strip or a gas-filled bellows that responds to temperature changes and adjusts the heating or cooling system accordingly. The temperature is set using a dial or a slider, which does not represent a discrete digital value.
Lastly, lightning itself is not a digital quantity. It is a natural atmospheric discharge of electricity. However, if you are referring to a lightning sensor or detector that measures and detects lightning strikes, it could involve digital quantities. Such sensors often use digital circuits and algorithms to analyze the electrical signals generated by lightning strikes and identify their characteristics.
Know more about Speedometer here:
https://brainly.com/question/20480446
#SPJ11
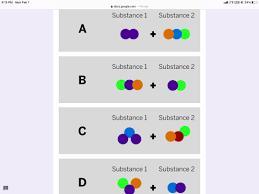
Starting substances
Substance 1
?
Substance 2
+ ?
Jamie works at a company that makes cleaning chemicals. She is trying to make a chemical that smells like flowers. She took two
samples that were gases at room temperature and mixed them in a sealed container.
The diagram above shows the repeating groups of atoms that make up the two starting substances.
After mixing, Jamie found two substances that smelled like flowers in the sealed container. (Nothing had escaped.)
Which of the diagrams shows the repeating groups of atoms that make up the ending substances?
Answers
The combination of the compounds can be found in option C
How is a compound formed?
A compound is formed through a chemical reaction or a combination of elements. In a chemical reaction, two or more elements combine or react with each other to form a compound.
The elements involved in the reaction undergo a rearrangement of their atoms and bonding to form new chemical bonds, resulting in the formation of a compound with different properties from the original elements. This is clear from the images that have been shown in the question.
Learn more about compound:https://brainly.com/question/14117795
#SPJ1

Someone pls help me

Answers
Answer:
A. 4 cm, 40 mm
B. 5.5 cm, 50 mm
C. 8.8cm, 80 mm
D. 8.2 cm, 82 mm
E. 9.5cm, 95 mm
Explanation:
NB: 1 cm = 10 mm
Which of these ions is most likely to be leached from the soil?
a. magnesium ions,
b. chlorine ions,
c. calcium ions,
d. iron ions
e. potassium ions
Answers
Which is the most complete charge balance expression for a saturated solution of cac2o4?
Answers
The most complete charge balance expression for a saturated solution of CaC₂O₄ is [Ca²⁺] = [C₂O₄²⁻]
Saturated solution is a solution that has dissolved as much solute as it is capable of dissolving. CaC₂O₄ is named as Calcium oxalate it is colourless solution. complete charge balance expression for a saturated solution of CaC₂O₄ are as follow:
CaC₂O₄ → 1Ca²⁺ + 1C₂O₄²⁻
Know more about saturated solution of CaC₂O₄
https://brainly.com/question/28500291
#SPJ4
what does the name cambrian period mean?
Answers
Answer:
the first geological time period of the Paleozoic Era
Explanation:
Determine the number of moles in 154g of Li2CO3
Answers
Given:
Weight of Li2CO3 = 154 g
To find:
number of moles of Lithium Carbonate Li2CO3 = ?
Solution:
first we have to calculate the molar mass of Lithium Carbonate,
The elemental composition of Li2CO3
Li = 2 units, Atomic weight of each Li atom is 7 g/mol
Li = 2 × 7 = 14 g/mol
C = 1 unit, Atomic weight of Carbon is 12 g/mol
O = 3 units, Atomic weight of each O atom is 16 g/mol
O = 3 × 16 = 48 g/mol
Atomic weight of Li2CO3 = 14+12+48 = 72 g/mol
now,
\(number \: of \: moles = \: \frac{Given \: weight \: of \: substance}{Atomic \: weight \: of \: substance} \)
Number of moles = 154/72 = 2.14 moles
Answer- The number of moles of Lithium Carbonate is 2.14
Thanks for joining brainly community.
what is the net ionic equation that occurs when zinc nitrate and lithium phosphate are mixed together in water? a precipitate of zinc phosphate is formed.
Answers
The net ionic equation that occurs when zinc nitrate and lithium phosphate are mixed together in water is 3Zn2+ + 2PO43- ⟶ Zn3(PO4)2(s)
When zinc nitrate and lithium phosphate are combined in water, a precipitate of zinc phosphate is created. It is an insoluble white solid. The compounds that are directly engaged in the chemical reaction are the only ones that are shown in the net ionic equation. Two soluble ionic chemicals are involved in the double replacement reaction.
In an aqueous solution, zinc nitrate, Zn(NO3)2, fully dissociates into zinc cations, Zn2+, and nitrate anions, NO3-.
In an aqueous solution, lithium phosphate (Li3PO4) fully dissociates into lithium cations (Li+) and phosphate anions (PO4)3-.
3Zn2+ + 2PO43- ⟶ Zn3(PO4)2(s)
To learn more about zinc nitrate click here https://brainly.com/question/29355805
#SPJ4
balance the following redox reaction in acidic solution. Zn(s) + MnO2(s) --> Zn2+ (aq) + Mn2+ (aq)
Answers
Zn(s) + 2H+(aq) + MnO2(s) --> Zn2+(aq) + Mn2+(aq) + H2O(l)
In this reaction, Zn(s) is oxidized to Zn2+(aq) and MnO2(s) is reduced to Mn2+(aq) in an acidic solution with the help of H+(aq) ions.
To balance the redox reaction in acidic solution, first, separate the reaction into half-reactions:
Zn(s) → Zn2+ + 2e-
MnO2(s) + 4H+ + 2e- → Mn2+ + 2H2O
Next, balance the atoms other than H and O in each half-reaction:
Zn(s) → Zn2+ + 2e-
MnO2(s) + 4H+ + 2e- → Mn2+ + 2H2O
Add H2O to the half-reaction that needs oxygen:
Zn(s) → Zn2+ + 2e-
MnO2(s) + 4H+ + 2e- → Mn2+ + 2H2O
Add H+ to the half-reaction that needs hydrogen:
Zn(s) → Zn2+ + 2e-
MnO2(s) + 4H+ + 2e- → Mn2+ + 4H2O
Multiply each half-reaction by a factor that makes the number of electrons equal:
Zn(s) → Zn2+ + 2e-
2MnO2(s) + 8H+ + 4e- → 2Mn2+ + 8H2O
Add the half-reactions together and cancel out common terms:
Zn(s) + 2MnO2(s) + 8H+ → Zn2+ + 2Mn2+ + 4H2O
Therefore, the balanced redox reaction in acidic solution is:
Zn(s) + 2MnO2(s) + 8H+ → Zn2+ + 2Mn2+ + 4H2O
Learn more about acidic solution here:-
https://brainly.com/question/13632841
#SPJ11
PLZ HELP WILL BRAINLIEST balance the following equation: N2 + H2 --> NH3
Write the coefficients that you decide to balance the equation like this 3, 4, 3. If you do not add a coefficient in front of an element or compound, use a 1 in your answer. For instance the for this balanced equation: 2H2 + O2 --> 2H2O you would write your answer: 2, 1, 2
Answers
Answer:
1,3,2
Explanation:
N2 + 3H2-----> 2NH3
An object has balanced forces acting on it. Which of the following describes the result these balanced forces have on the object? A. It remains at rest or speeds up in the same direction. B. It speeds up in the same direction or moves at constant speed. C. It speeds up in the same direction or slows down. D. It remains at rest or moves at constant speed in the same direction.
Answers
Answer:
C.It remains at rest or moves at constant speed in the same direction.
Explanation:
First, remember the Newton's 1st law of motion which states that the object at rest will remain at rest and that in motion will stay in motion with the same speed and same direction unless acted by unbalance forces.
Balanced forces on an object occur when two forces at act on an object are equal in size and act in opposite direction. In this case, a stationary object will stay at rest while an object moving will continue to move at the same speed and same direction.
An object acted by balanced forces is said to be at equilibrium, thus the state of motion will be maintained.The object will not accelerate. A good example of an object acted by balanced forces is an object at rest or in constant motion such as a car that stopped at red-light signal or a car travelling at a constant speed.
Dogada gerceklesen kimyasal tepkimeler ornekler
Answers
Answer:
Combustion (burning) of wood.
The metabolism of food in the body.
Cooking an egg
Digesting sugar with the amylase in saliva.
Explanation:
Here are some example, I hope this helped!
(Ahşabın yanması (yanması).
Vücuttaki gıda metabolizması.
Yumurta pişirmek
Tükürükteki amilaz ile şekeri sindirmek.)
What is the speed of a cheetah that runs 30 miles in 0.5 hours?
a
Answers
Answer:
60 mph (miles per hour)
Explanation:
0.5 hours is 1/2 of an hour, so to get the number of miles for a whole hour you multiply the miles ran by 2.
30 times 2 is 60.
S = 30/0.5 = 60mi/ hr
One type of atomic particle that is found in the nucleus does not contribute to an elements atomic number what are two characteristics of this type of atomic particle
Answers
This particle is called neutron. It's characteristics are:
1. Neutron is electrically.
2. They show magnetic properties
Name the most active earthquake zone.
Whe
Answers
Answer:
Pacific Ring of Fire.
Explanation:
Japan sits in one of the most active earthquake zones on the planet: the Pacific Ring of Fire.
Hope this helps you. :)
Answer: i would say Indonesia.
Explanation:
If 3.4 atm of a gas at 644.4 K is compressed to 11.6 atm at constant volume. What is the new pressure of the gas?
Answers
The temperature in the final pressure formation is 2201.13K and the change in the pressure is 8.2atm
It is given that the initial pressure of the gas P₁ is 3.4atm and the initial temperature is T₁ 644.4K the final pressure of the gas is 11.6 atm. To find the final temperature, According to Gay Lussac's Law,
P₁/T₁ = P₂/T₂
On substituting the values of P₁,T₁ and T₂ we get,
3.4/644.4 = 11.6/T₂
0.005276 = 11.6/T₂
T₂ = 11.6/0.005276
T₂ = 2201.13K
Therefore the final temperature T₂ is 2201.13K when the pressure is changed from 3.4 atm to 11.6 atm
Thus the pressure change is calculated by,
ΔP = P₂ - P₁
ΔP = 11.6 - 3.4
ΔP = 8.2 atm
The change in pressure is 8.2atm
To know more about Charles's Law click below:
https://brainly.com/question/16927784
#SPJ9
25. The half-life of radioactive strontium- 90 is 29 years, In 1960, radioactive strontium-90 was released into the at. mosphere during testing of nuclear weapons, and was ab. sorbed into people's bones. How many years does it take
Answers
It takes approximately 100.704 years (since 1964) until only 9 percent of the original amount of radioactive strontium-90 absorbed remains.
The half-life of radioactive strontium-90 is given as 29 years, which means that every 29 years, the amount of radioactive strontium-90 is reduced by half.
To find the number of years it takes until only 9 percent of the original amount remains, we can set up the following equation:
(0.5)^(t/h) = 0.09
Where:
t represents the number of years since 1964 (the initial time),
h represents the half-life of 29 years, and
0.09 represents 9 percent.
Let's solve for t:
(0.5)^(t/29) = 0.09
Taking the natural logarithm (ln) of both sides:
ln[(0.5)^(t/29)] = ln(0.09)
Using the logarithmic property: ln(a^b) = b × ln(a):
(t/29) × ln(0.5) = ln(0.09)
Dividing both sides by ln(0.5):
t/29 = ln(0.09) / ln(0.5)
t = 29 × (ln(0.09) / ln(0.5))
Using a calculator, we can find the value of t:
t = 29 × (-2.40794561 / -0.69314718)
t = 100.704
Learn more about radioactive -
brainly.com/question/23759636
#SPJ11
Elements make up all things. What is it called
Answers
Answer:
Matter
Explanation:
All matter is made up of substances called elements
WILL GIVE 50 POINTS AND BRAINLIEST!!!!!
Co^3+ and BrO^- what is the empirical formula and name? K^+ and C2H3O2^- what is the empirical formula and name of compound?
Answers
formula : Co(BrO)3
2. name: potassium acetate
formula: KC2H3O2
what is the chemical formula of the ionic compound formed along with water in the reaction of chlorous acid and barium hydroxide?
Answers
Barium chloride and water are created when barium hydroxide and hydrochloric acid combine.
what is ionic compound?
An ionic compound in chemistry is a chemical complex made up of ions kept together by electrostatic forces known as ionic bonding. Although the chemical is generally neutral, it does include positively charged ions known as cations and negatively charged ions known as anions. Simple ions like sodium (Na+) and chloride (Cl) in sodium chloride or polyatomic species like the ammonium (NH+ 4) and carbonate (CO2 3) ions in ammonium carbonate can be present in these compounds. Since each individual ion in an ionic compound typically has many nearest neighbours, they aren't thought of as being a part of molecules at all but rather as a continuous three-dimensional network. When solid, ionic chemicals often take the shape of crystals.
The balanced equation for this reaction is: 2HCl (aq) + Ba (OH)2 (aq) → BaCl2 (aq) +2H2 0 (1) If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid.
to learn more about ionic compounds follow the given link: https://brainly.com/question/13539062
#SPJ4
difference between very short and Short period in modern periodic table
Answers
Answer:
There are three types of periods in the modern periodic table: very short periods, short periods, and long periods.
Very short period contains only two elements, Hydrogen and Helium. These elements have only one shell, and their electrons can only occupy the s-orbital.Short periods contain eight elements. The first two elements in a short period can only occupy the s-orbital, while the remaining six elements can also occupy the p-orbital.Long periods contain 18 elements. The first six elements in a long period can only occupy the s- and p-orbitals, while the remaining 12 elements can also occupy the d-orbital.The difference between very short periods and short periods is the number of elements they contain. Very short periods only contain two elements, while short periods contain eight elements. The difference between short periods and long periods is the number of orbitals that can be occupied by electrons in each period. Short periods can only have electrons in the s- and p-orbitals, while long periods can also have electrons in the d-orbital.
Here is a table summarizing the differences between very short periods, short periods, and long periods:
Period type: Very short periodNumber of elements: 2
Orbitals that can be occupied by electrons: s-orbital only.Period type: short period
Number of elements: 8
Orbitals that can be occupied by electron: s- and p-orbitals.Period type: long period
Number of element: 18
Orbitals that can be occupied by electrons: s-, p-, and d-orbitals