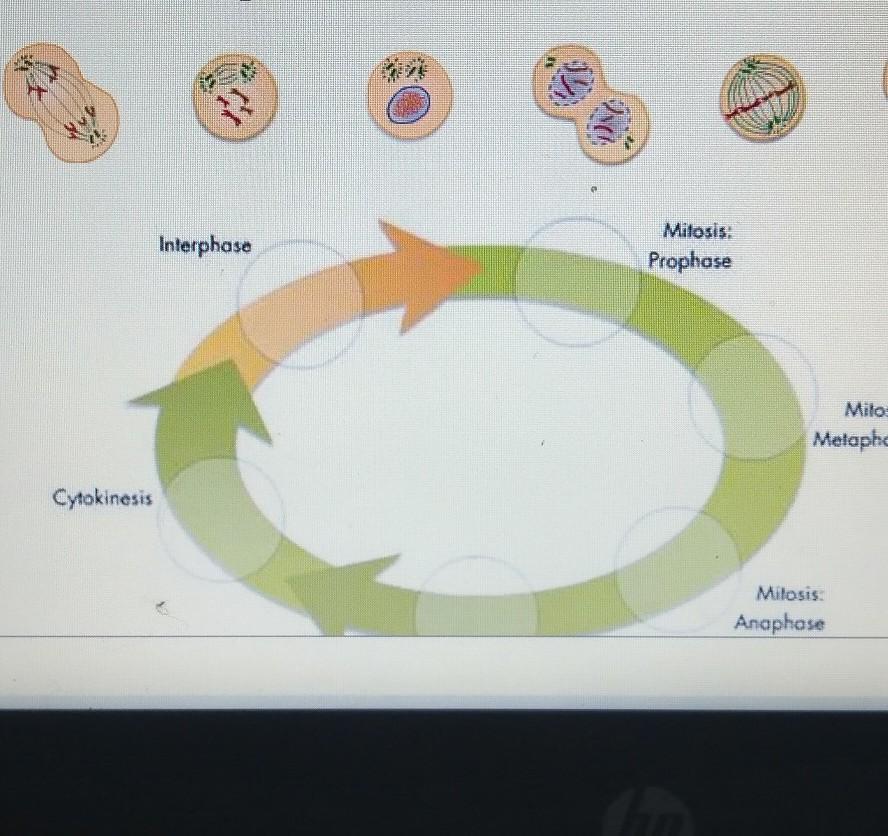
Interphase Milosis: Prophese Mitosis: Melaphese Cytokinesis Anaphose
drag and drop
Answers
Related Questions
What mass of copper metal will be formed when excess magnesium metal is reacted with:
(a) 250.0 mL of a 1.0 M copper M chloride solution (b) 100.0 mL of a 0.25 M copper (II) chloride solution
Answers
Answer:
A
Explanation:
HELP PLEASE ! Where do oil and natural gas come from? Make sure to include information about
the rock cycle, the history of life on Earth, fossils, and how fossils form.

Answers
Natural gas is found in rock formations deep below the surface of the Earth. Petroleum, or oil, is often found in the same areas.
Why does a Lewis structure of a covalent molecule show only the valence electrons?
Answers
Answer:
Only the valence shell are shown in the Lewis electron dot structures because, the chemical reactivity of an atom of an element is determined by the valence electrons of the atom and not by all the electrons of the atom including the inner electrons.
Explanation:
Lewis electron dot structures are structures that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
In the Lewis symbol for an atom, the chemical symbol of the element is written, and the valence electrons of the atom are represented as dots surrounding it. Only the electrons in the valence shell are shown using the Lewis electron dot structures.
Lewis structures for molecules show each atom and its position in the structure of the molecule using its chemical symbol. Dots or lines are drawn between atoms to show the electrons that are involved in the chemical bonding between the atoms in the molecule. Valence electrons in each atom that are not involved in bonding known as lone pairs, are represented as pairs of dots, and are placed next to each atom bearing the electron.
Electrons that are not in the valence level are not shown in the Lewis electron dot structures . This is because, the chemical reactivity of an atom of an element is determined by the valence electrons of the atom and not all the electrons of the atom including the inner electrons.
What happens in the nucleus when a lithium atom becomes an ion
Answers
Answer:
An atom that gains a negative electron, it becomes a negative ion. ... A lithium atom has 3 protons and 3 electrons. It can lose one of its electrons, making it an ion. It now has more positive protons than electrons so it has an overall positive charge.
Answer:
This means a Lithium atom formed into a Lithium ion by losing one of its electrons. It has three electrons and three protons to begin with. So when it loses or gains one the amount isnt equal, that is why it becomes an ion. If it gained electrons it would have a negative charge on the nulcleus, though if it lost electrons it would have a positivec charge. Hope this helped!!
In what way are the transition metals different than the alkali metals and alkaline earth metals?
Answers
Transition metals are different than alkali metals and alkaline earth metals because transition metals are more dense but much less reactive as compared to these metals.
As compared to transition metals, alkali, and alkaline earth metals are less dense because these metals of the first two groups are found to have higher atomic volumes. Therefore the transition metals have higher masses than their corresponding alkali metals as well as alkaline earth metals.
In addition to this, compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are found to be much less reactive which is due to their high ionization potential and melting temperature.
To learn more about transition metals; click here:
https://brainly.com/question/12843347
#SPJ4
the cori cycle is a metabolic pathway that involves active muscles and the liver. in the liver, fatty acids react to form c o 2 and h 2 o, releasing a t p, and lactate uses the a t p to form glucose in a process called gluconeogenesis. the glucose moves to the muscles to form lactate, releasing a t p, a process called glycolysis. the lactate then moves from the muscles to the liver to complete the cycle. complete the sentences about the cori cycle. you are currently in a labeling module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop. muscles break down into , which undergoes glycolysis. the end product of glycolysis in active muscles is , which is transported in the blood. the liver uses energy from to drive . the produced in the liver is transported to the muscle in the bloodstream. answer bank
Answers
The muscles break down glycogen into glucose which undergoes glycolysis . the end product of glycolysis in active muscles is lactate, which is transported in the blood. the liver uses energy from fally acid oxidation drive glucose. the glucose produced in the liver is transported to muscle in the bloodstream.
muscles breaks into glycogen and glucose. the lactate is always the end product in glycolysis. by the fally acid oxidation liver uses energy. the cori cycle is the metabolic pathway which involves the active muscles and the liver. so, the fatty acid will react and it forms the carbon dioxide and the water.
To learn more about glycolysis here
https://brainly.com/question/16004094
#SPJ4
22. How many atoms are there in 344.75 g of gold nugget? a. 1.05 x 10 to the power of 24 atoms b. 1.05 x 10 to the power of 23 atoms c. 6.02 x 10 to the power of 23 atoms d. 197 atoms Is B the answer
Answers
Answer:
1.053×10²⁴ atoms of gold
Explanation:
Hello,
Gold nugget are usually the natural occurring gold and they contain 85% - 90% weight of pure gold.
In this question, we're required to find the number of atoms in 344.75g of a gold nugget.
We can use mole concept relationship between Avogadro's number and molar mass.
1 mole = molar mass
Molar mass of gold = 197 g/mol
1 mole = Avogadro's number = 6.022 × 10²³ atoms
Number of mole = mass / molar mass
Mass = number of mole × molar mass
Mass = 1 × 197
Mass = 197g
197g is present in 6.022×10²³ atoms
344.75g will contain x atoms
x = (344.75 × 6.022×10²³) / 197
X = 1.053×10²⁴ atoms
Therefore 344.75g of gold nugget will contain 1.053×10²⁴ atoms of gold
As per the atoms the 344.5 g of gold nuggets have a 1.05x10 and have a power of 24 atoms.
Gold nugget are usually the natural occurring gold and they contain 85% - 90% weight of pure gold. In this question, we're required to find the number of atoms in 344.75g of a gold nugget. 1.053×10²⁴ atoms of gold.Learn more about the atoms are there.
brainly.com/question/18510197.
QUESTION 1.
Element X on Planet Qatar has three known isotopes: X-121 with a relative abundance of 43.2%, X-123 with a relative abundance of 53.1%, and X-129 with a relative abundance of 3.70%. What is the average atomic mass in amu of Element X given this information?
QUESTION 2.
How did Rutherford's experiment change the way scientists had previously viewed atomic structure? How did this pave the way for subsequent changes up to and including the currently accepted quantum mechanical atomic model?
QUESTION 3.
How is percent abundance related to average atomic mass?
Answers
a) The percentage abundance determines the relative atomic mass.
b) The relative atomic mass for the element X is122.5 amu
c) The Rutherford's model explained showed the movement of the electrons.
What is the relative abundance?The isotopes are the kinds of atoms of an element that we can see. We know that all the element that we have in the periodic table are composed of the isotopes and the isotopes of the elements are important in the determination of the relative atomic mass of the element that we may be considering here called X
We can now be able to see from the forgoing that we have here that;
Relative atomic mass = Weighted average of all the isotopes
As such we have;
(121 * 0.432) + (123 * 0.531) + (129 * 0.0370)
52.27 + 65.31 + 4.773
=122.35 amu
The Rutherford model of the atom can also be called the planetary model of the atom and this is the model that can be able to explain to us the way that the electrons move round the nucleus.
Learn more about atoms:brainly.com/question/13654549
#SPJ1
Can someone help please?
Copper occurs naturally as a mix of two isotopes: Copper-63 with a mass of 62.930 amu and copper-65 with a mass of 64.928 amu. The percent abundance of copper-63 is 69.09%.
Calculate the atomic mass of copper.
Answers
Answer: 63.55
Explanation:
The attached chart shows the calculation. The orange line points to the % abundance of Cu-65, which is the difference of 100% less the % Abundance of Cu-65 (since there are only 2 isotopes).
The weighted average contribution of each istope is calculated (blue arrow) and then summed to find the atomic mass of copper.

Assume we have 759 liters of N, at ST. What is the mass of the nitrogen gas? Give answers to the nearest whole number.
Answers
So, at STP, there are around 891 grammes of nitrogen gas in every 759 litres.
Which mass is greater, 14 or 15?The two stable isotopes of naturally occurring nitrogen (7N) are nitrogen-14 and nitrogen-15, with nitrogen-14 constituting 99.6% of all naturally existing nitrogen. Along with one nuclear isomer, 11mN, fourteen radioisotopes with atomic masses ranging from 10 to 25 are also known.
Assuming "ST" refers to standard temperature and pressure (0°C and 1 atm), we can use the ideal gas law to calculate the mass of nitrogen gas:
PV = nRT
where n is the number of moles, P is the pressure, V is the volume, and T is the temperature, and R is the gas constant.
The temperature and pressure are 273.15 K and 1 atm, respectively, at STP.
To solve for n, the number of moles, we can rearrange the ideal gas law equation as follows:
n = PV / RT = (1 atm) * (759 L) / (0.0821 L·atm/(mol·K) * 273.15 K) = 31.8 mol
Now we can calculate the mass of nitrogen gas:
mass = n * molar mass = 31.8 mol * 28.01 g/mol ≈ 891 g.
To know more about nitrogen gas visit:-
https://brainly.com/question/13907528
#SPJ1
if 106.66 grams of o2 are reacted how many particles of h2o are produced
Answers
106.66 grams of O2 reacting produces 4.01 x 10^24 particles of H2O.
StepsWe can start by balancing the chemical equation for the reaction between oxygen (O2) and hydrogen (H2) to form water (H2O):
2H2 + O2 → 2H2O
The balanced equation tells us that 1 mole of O2 reacts with 2 moles of H2 to form 2 moles of H2O.
To determine the number of particles of H2O produced from 106.66 grams of O2, we need to convert the mass of O2 to moles using its molar mass:
Molar mass of O2 = 32.00 g/mol
Number of moles of O2 = mass / molar mass
Number of moles of O2 = 106.66 g / 32.00 g/mol
Number of moles of O2 = 3.33 mol
Since the balanced equation tells us that 1 mole of O2 reacts with 2 moles of H2O, we can use stoichiometry to determine the number of moles of H2O produced:
Number of moles of H2O = (number of moles of O2) x (2 moles of H2O / 1 mole of O2)
Number of moles of H2O = 3.33 mol x 2
Number of moles of H2O = 6.66 mol
Finally, we can use Avogadro's number to convert the number of moles of H2O to the number of particles:
Number of particles of H2O = (number of moles of H2O) x (6.02 x 10^23 particles/mol)
Number of particles of H2O = 6.66 mol x 6.02 x 10^23 particles/mol
Number of particles of H2O = 4.01 x 10^24 particles
Therefore, 106.66 grams of O2 reacting produces 4.01 x 10^24 particles of H2O.
learn more about Avogadro's number here
https://brainly.com/question/1513182
#SPJ1
0.15 gm of metallic oxide was dissolved in 100 ml of 0.1 N H2SO4 and 25.8 ml of 0.095N NaOH were used to neutralise the remaining H2SO4.Calculate the equivalent weight of metallic oxide and metal.
Ans: metallic oxide=19.87
metal=11.87
Answers
Answer:
Explanation:
25.8 ml of .095 N NaOH is needed to neutralise the remaining acid
equivalent of NaOH used = 25.8 x .095 / 1000 = .002451 gm equivalent .
acid remaining = .002451 gm equivalent .
acid initially taken = 100 ml of .1 N / 1000 = . 01 gm equivalent
acid reacted with metal = .01 -.002451 = .007549 gm equivalent
This must have reacted with same gram equivalent of metal oxide
.007549 gm equivalent = .15 gm of metal oxide
1 gm equivalent = 19.87 gm
equivalent weight of metal = 19.87 - equivalent weight of oxygen
= 19.87 - 8 = 11.87 .
1
Are two atoms of the same element identical??
Answers
why is water an excellent solvent for most ionic compounds and polar covalent molecules but not for non-polar compounds?
Answers
Water is an excellent solvent for most ionic compounds and polar covalent molecules but not for non-polar compounds because of its content loaded with certain chemical properties that make it a highly effective solvent. Water molecules are polar, and due to their dipolar nature, the oxygen atom carries a negative charge while the hydrogen atoms carry a positive charge.
The polarity of the water molecule allows it to interact with and dissolve other polar and ionic substances.When an ionic compound is dissolved in water, the ions of the compound dissociate into individual charged species (cations and anions), and these charged species are solvated by water molecules. The polarity of the water molecule allows it to interact with the ions by attracting the positively charged ions to the negative end of the water molecule and vice versa.
The same is true for polar covalent molecules, which have a net dipole moment. Water molecules can interact with these molecules, forming a solvation layer around them. On the other hand, non-polar compounds lack a net dipole moment, so they don't interact with the water molecules. Instead, non-polar compounds interact with each other via van der Waals forces, making it more challenging for them to dissolve in water.
To know more about ionic compounds, visit:
https://brainly.com/question/30420333
#SPJ11
what is the percent composition of nitrogen in sodium nitride (nan3)?
Answers
The percent composition of nitrogen in sodium nitride is approximately 16.87%.
How to find the percentage compositionThe percent composition of nitrogen in sodium nitride (Na₃N) can be calculated by determining the molar mass of nitrogen and the molar mass of the compound as a whole.
molar mass
molar mass of nitrogen = 14.01 grams
molar mass of sodium nitride = (3 * molar mass of Na) + (1 * molar mass of N)
molar mass of sodium nitride = (3 * 22.99 g/mol) + (1 * 14.01 g/mol)
molar mass of sodium nitride = 82.98 g/mol
percent composition of nitrogen
Percent composition of nitrogen = (molar mass of N / molar mass of Na₃N) * 100
Percent composition of nitrogen = (14.01 g/mol / 82.98 g/mol) * 100
Percent composition of nitrogen = 16.87%
Therefore, the percent composition of nitrogen in sodium nitride is approximately 16.86%.
Learn more about percent composition at
https://brainly.com/question/17021926
#SPJ4
if households choose to take some fraction of each check they deposit and hold it as currency, then the simple deposit multiplier the real-world multiplier.a. is equal to b. bears no relationship to c. is less than d. is greater than
Answers
The simple deposit multiplier is higher than the real-world money multiplier if households decide to keep some portion of each check they deposit in their possession as money.
For each unit of money a bank has in reserves, the deposit multiplier determines the most money that can be made. The portion of the deposit amount that can be lent out is represented by the deposit multiplier. The Federal Reserve's reserve requirement typically determines that proportion of real-world money multiplier.
The process by which banks lend money and the outcome is more money flowing in the economy is referred to as a "real-world money multiplier." That is, the amount of money is increased.
Learn more about deposit multiplier here
https://brainly.com/question/14279576
#SPJ4
Which is the balanced equation for S8 + O2 → SO2?
S8 + O16 → 8SO2
S8 + O2 → S8 + O2
S8 + O2 → S8O2
S8 + 8O2 → 8SO2
Answers
Answer:
The answer is A S8 + O16 → 8SO2
Explanation:
The balanced chemical equation is -:
S₈ + O₂-----> 8SO₂ therefore (d) is correct .
What do you mean by balanced chemical equation ?A balanced chemical equation is an equation that has equal numbers of atoms for each element both on the reactant and product sides.
Methods to balance the chemical equation -:
The number of atoms for a given element is calculated by multiplying its coefficient with the element’s subscript in its chemical formula.
The coefficients in front of the formulas to show how many molecules of that material are used or produced.
Hence , the balanced chemical equation is -:
S₈ + O₂-----> 8SO₂ ,hence option (d) is correct .
Learn more about balanced chemical equation, here:
https://brainly.com/question/12192253
#SPJ6
In a metallic bond, electrons _____.
⚪ are shared
⚪ move from a high energy level to a low energy level within one atom
⚪ are completely transferred between bonded atoms
⚪ move freely between the clouds of several atoms
Answers
Answer:
They move freely between the clouds of several atoms, si the correcto Answer is D
Explanation:
Hope this helps.
list 3 ways to increase the solubility of a liquid
Answers
Answer:
Three ways I can come up with are increasing the temperature, increased the amount of solvent, and using a solvent with similar polarity as the solute.
Explanation:
the formula for caffeine is c8h10n4o2. how many total atoms are in 0.75 moles of caffeine
Answers
In 0.75 moles of caffeine, there are a total of 6 carbon atoms, 7.5 hydrogen atoms, 3 nitrogen atoms, and 1.5 oxygen atoms.
To determine the total number of atoms in 0.75 moles of caffeine, we need to consider the molecular formula of caffeine, which is C8H10N4O2. The molecular formula provides the ratios of each element present in the compound. By multiplying the number of atoms in each element by the corresponding coefficient in the molecular formula, we can calculate the total number of atoms. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms in each molecule of caffeine. Multiplying these values by 0.75 moles will give us the total number of atoms in 0.75 moles of caffeine.
The molecular formula of caffeine, C8H10N4O2, provides the number of atoms for each element present in one molecule of caffeine. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms.
To calculate the total number of atoms in 0.75 moles of caffeine, we need to multiply the number of atoms for each element by the coefficient in the molecular formula, and then multiply that by the number of moles (0.75 moles).
For carbon (C): 8 atoms x 0.75 moles = 6 atoms (since there are 8 carbon atoms in one molecule of caffeine).
For hydrogen (H): 10 atoms x 0.75 moles = 7.5 atoms (since there are 10 hydrogen atoms in one molecule of caffeine).
For nitrogen (N): 4 atoms x 0.75 moles = 3 atoms (since there are 4 nitrogen atoms in one molecule of caffeine).
For oxygen (O): 2 atoms x 0.75 moles = 1.5 atoms (since there are 2 oxygen atoms in one molecule of caffeine).
To learn more about molecular click here:
brainly.com/question/156574
#SPJ11
When rocks break down or decompose, they can form
A.
soil.
B.
magma.
C.
bigger rocks.
D.
lava.
Answers
Answer:
C
Explanation:
because when rocks break down they can form and once they form they can make more and even bigger rocks hopes this helps.
11. A chemical reaction happens when chemicals combine to form a new _______
Answers
Answer:
substances
Explanation:
A chemical reaction happens when substances break apart or combine to form one or more new substances.
^Hope it helps, Hazel^
Assuming no other changes in temperature or volume, what will be the effect on the pressure inside a container of gas if the identity of gas is changed from nitrogen to helium
Answers
The pressure will increase if the gas is changed from nitrogen to helium.
Kinetic theory of gasesThe question is incomplete but I will try to help the much I can. We must note that the kinetic energy of the molecules of a gas depends on the temperature and the molar mass of the gas.
Since the average velocity of a gas depends on molar mass also, the pressure will increase if the gas is changed from nitrogen to helium.
Learn more about gases: https://brainly.com/question/1369730
Which of these limiting factors is MOST likely to affect low-growing plants in a tropical forest? Group of answer choices air water shelter sunlight
Answers
Answer:
air, water, sunlight
Explanation:
A limiting factor may be defined as a environmental condition or a resource that can limit the plant growth as well as distribution or the abundance of an organism or its population within the ecosystem. The ability of any plant species to grow and spread throughout any geographic area is the direct result of the adaption to its biotic and abiotic components of that region.
Some of the factor that affect the plant growth are : sunlight, air, proper temperature, moisture, nutrients, wind,etc.
The lack of the any one of the above essential component will determine the health of the plant.
Calculate the vapor pressure (in torr) of a 35°C solution of ethanol and propanol where the mole fraction of propanol is .75.
Assume ideal behavior.
P°ethanol = 100 torr
P°propanol = 36 torr
Answers
Answer:
i wish i know
Explanation:
As the reaction in a galvanic cell proceeds towards products, which of the following are true?
A) ΔG starts at 0, stays same
B) ΔG starts < 0, becomes more negative
C) ΔG starts < 0, stays same
D) ΔG starts < 0, becomes more positive
E) ΔG starts > 0, stays same
Answers
In a galvanic cell, the reaction proceeds towards the production of products. ΔG starts < 0, becomes more negative
Option B is correct .
As the reaction proceeds, the Gibbs free energy (ΔG) reduces, and the following are true: ΔG starts < 0, becomes more negative.
When the reaction in a galvanic cell proceeds towards the production of products, the Gibbs free energy starts with a negative value, and it becomes even more negative.
The Gibbs free energy (ΔG) is a measure of the available energy in a system that can be used to do work. It measures the difference between the free energy of the final state and the initial state.The Gibbs free energy change of a system is dependent on the enthalpy and entropy change. If the enthalpy change is negative (exothermic), and the entropy change is positive (disorderly), the Gibbs free energy change is negative, and the reaction is spontaneous.
Learn more about Gibbs energy :
brainly.com/question/13765848
#SPJ11
Which of the tests would involve a chemical change in the mineral?
Answers
hmmm water
Explanation:
Water can change if you put food coloring
in it
Choose the compound that should have the lowest melting point according to the ionic bonding model.
Group of answer choices
BaS
BaCl2
Fe2O3
MgS
Answers
Among the given compounds, MgS should have the lowest melting point according to the ionic bonding model.
These oppositely charged ions are attracted to one another, resulting in the formation of an ionic bond.The temperature at which a solid melts to form a liquid is known as the melting point of a substance. This is a physical property that varies between different compounds.
The strength of the ionic bond between two ions is inversely related to the distance between them. As a result, compounds with stronger ionic bonds have higher melting points, while those with weaker ionic bonds have lower melting points.
In this case, MgS should have the lowest melting point because it has the weakest ionic bond among the given compounds.
This is because both magnesium and sulfur are relatively small atoms that form ions with a +2 charge and a -2 charge, respectively. As a result, the ions in MgS are smaller and have a weaker attraction to one another than the other compounds, resulting in a lower melting point.
To know more about physical property click on below link:
https://brainly.com/question/31131341#
#SPJ11
Porosity is the fraction of material occupied by empty spaces. It can be expressed as a percent or a decimal. Assume that the porosity of a sample of sand and a sample of gravel is the same: 30 percent (0.30). Determine the porosity of a mixture of the sand and gravel, assuming that the sand fills the spaces between the gravel
Answers
Assuming that the porosity of both the sand and the gravel samples is the same at 30%, the remaining space in each sample (or the solid volume fraction) would be 70%.
When the sand and gravel are mixed together, the sand will fill the spaces between the gravel. The total volume of the mixture will be the sum of the volumes of the sand and gravel.
Let's assume we have a certain volume of gravel, and we add sand until the spaces between the gravel are completely filled. The total volume of the mixture will be greater than the volume of the gravel alone, because the sand has filled in the spaces between the gravel.
The porosity of the mixture will be the fraction of the total volume that is empty space. Since the sand has filled the spaces between the gravel, the porosity of the mixture will be less than 30%.
The exact porosity of the mixture will depend on the relative proportions of sand and gravel in the mixture, as well as the shape and size of the particles. However, we can estimate the porosity of the mixture based on the assumption that the sand fills the spaces between the gravel. If we assume that the volumes of sand and gravel are equal, then the porosity of the mixture will be:
Porosity of mixture = (Volume of empty space in mixture) / (Total volume of mixture)
Total volume of mixture = Volume of sand + Volume of gravel
If the volumes of sand and gravel are equal, then the total volume of the mixture will be twice the volume of either the sand or the gravel. Therefore:
Total volume of mixture = 2 x Volume of sand (or gravel)
Volume of empty space in mixture = Volume of gravel x Porosity of gravel + Volume of sand x Porosity of sand
Since the porosity of the sand and gravel samples are assumed to be the same at 30%, we have:
Volume of empty space in mixture = Volume of gravel x 0.30 + Volume of sand x 0.30
Substituting 2 x Volume of sand for the total volume of the mixture, we get:
Porosity of mixture = (Volume of gravel x 0.30 + 2 x Volume of sand x 0.30) / (2 x Volume of sand)
Simplifying the expression, we get:
Porosity of mixture = (Volume of gravel + 0.6 x Volume of sand) / (2 x Volume of sand)
So, the porosity of the mixture depends on the relative volumes of sand and gravel. If the volumes are equal, then the porosity of the mixture will be:
Porosity of mixture = (Volume of gravel + Volume of sand x 0.6) / (2 x Volume of sand)
For example, if we have 1 liter of sand and 1 liter of gravel, then the total volume of the mixture will be 2 liters. The volume of empty space in the mixture will be:
Volume of empty space in mixture = 1 liter x 0.30 + 1 liter x 0.30 = 0.6 liters
Therefore, the porosity of the mixture will be:
Porosity of mixture = 0.6 liters / 2 liters = 0.30 or 30%
To know more about relative volumes , visit :
https://brainly.com/question/2467054
#SPJ1
5. Water, wind, ice, and gravity are important agents of ___ which is a destructive force