Answers
Answer:
A
Explanation:
For some reason its this I did not read the explanation but its correct
Related Questions
50 Points (Brainliest too)! Please help!
1. In detail, explain what transmutation is.
2. In one to two sentences, explain what an alpha particle is.
3. Explain the difference between a chemical equation and a nuclear equation.
4. What is the relationship between the atomic number and the mass number of a 5. nucleus?
Please help me understand these!! I'm really confused!
Answers
Answer:
Transmutation is the conversion of an atom of one element into an atom of a different element through nuclear changes.
An alpha particle is a particle composed of 2 protons and 3 neutrons. It results from transmutation because of the change in protons in a large nucleus.
A chemical equation is balanced accord to the number of atoms of each element before and after the change. This is also shows the Law of Conservation of Matter.
A nuclear equation is balanced according to mass number and charge (atomic number). These equations can’t be balanced like chemical equations because the identities of the atoms can change. On the left of equation, the top number is the atomic mass and the bottom number is the atomic number. Take note that if you add the atomic mass (top number), you’ll get the the atomic mass of of the atom pre-transmutation. Same applies with atomic number. This shows a balanced nuclear equation.
The atomic number is the number of protons in a nucleus. The mass number is the sum of the protons and neutrons in a nucleus. Electrons have very small masses, so they are not accounted in atomic mass.
If we assume that the He nucleus is a sphere, its diameter measures approximately 2.0 fm. What is the density of the nucleus in g/cm3? Please provide your answer in grams per cubic centimeter.
Answers
The density of the He nucleus is approximately 2.8 × 10^17 grams per cubic centimeter (g/cm³).
To calculate the density of the He nucleus, we need to know its mass and volume. Given that the diameter of the nucleus is approximately 2.0 fm, we can calculate its radius as 1.0 fm (since the radius is half the diameter).
The volume of a sphere can be calculated using the formula V = (4/3)πr³, where V is the volume and r is the radius. Substituting the values, we get:
V = (4/3)π(1.0 fm)³
Using the conversion factor that 1 fm = 1 × 10^-13 cm, we can convert the volume from fm³ to cm³:
V = (4/3)π(1.0 × 10^-13 cm)³
Simplifying the equation further, we have:
V = (4/3)π(1.0 × 10^-13)³ cm³
Now, to calculate the density, we need the mass of the He nucleus. The mass of a He nucleus is approximately 4 atomic mass units (4 amu).
Using the conversion factor that 1 amu = 1.66 × 10^-24 g, we can convert the mass from atomic mass units to grams:
Mass = 4 amu × (1.66 × 10^-24 g/amu)
Now we have both the mass and volume. The density (D) of an object is defined as mass divided by volume:
D = Mass/Volume
Substituting the values, we get:
D = (4 amu × 1.66 × 10^-24 g/amu) / [(4/3)π(1.0 × 10^-13)³ cm³]
Simplifying the equation and performing the calculations, the density of the He nucleus is approximately 2.8 × 10^17 g/cm³.
For more such questions on density visit:
https://brainly.com/question/26364788
#SPJ8
An empty graduated cylinder weighs 25.489 g. When the cylinder contains 45.3 mL of an
unknown liquid,
it weighs 57.847 g. What is the mass of the unknown liquid? Show your work.
Answers
Answer:
In the given question, the mass of empty slender is given 25 points 489 Grand. The mass of Slender plus unknown liquid is given 57 points 847 g. The volume of a non liquid is given 45 three ml. We have to find the density of a non liquid. Firstly we will find the mass of unknown liquid. As the mass of unknown liquid is equal to the mass of cylinder plus unknown liquid minus the mask of empty cylinders. So the mass of a non liquid is equal to seven points 847 g -25 points 489 g. The mass of a non liquid will be 32 points 358 g. The formula to calculate the density of a non liquid is equal to the mass of a non liquid divided by the volume of a non liquid. So we will put the values as the mass of a non liquid is 32.358 g, Divided by the volume, which is given 45.3 ML. When we solve this Comes out to be 0.71 g, but I am in. So the Final answer is the density of a non liquid is equal to 0.71 g.
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
How many electrons are in an atom of elemental sodium?
equal to the number of neutrons
equal to the number of protons
equal to the number of protons and neutrons
Answers
Answer:
its the second option
equal to the number of protons
Explanation:
took the test
Answer:
equal to the number of protons
Explanation:
2021 edge
9. When 1.10 g of magnesium reacted with unlimited amount of HCl, the products were
hydrogen gas and magnesium chloride. What volume of hydrogen gas would be collected
if the reaction had been run at STP?
Answers
As per the balanced equation of the reaction one mole of Mg give s one mole of H2. 1.10 g of magnesium is 0.045 moles. Thus it gives 0.045 moles of H2 which is 1.02 litres.
What is magnesium chloride?Magnesium chloride is an ionic compound formed by the reaction of magnesium metal with two moles of hydrochloric acid. The reaction also produce one mole hydrogen gas.
The atomic mass of magnesium is 24 g/mol. Thus number of moles in 1.10 g is 1.10/24 = 0.045 moles.
As per the reaction equation one mole of magnesium gives one mole of hydrogen gas. Thus 0.045 moles of magnesium gives 0.045 moles of hydrogen gas.
At STP, the volume of one mole of any gas will be equal to 22.4 L. Thus volume of 0.045 moles of hydrogen at STP is:
volume = 0.045 × 22.4
=1.02 L.
Hence, the volume of hydrogen produce here is 1.02 L.
To find more on magnesium, refer here:
https://brainly.com/question/1533548
#SPJ1
What is most likely an example of a chemical reaction
Answers
Answer:
Chemical reactions often involve color changes, temperature changes, gas production, or precipitant formation. Simple examples of everyday reactions include digestion, combustion, and cooking. Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen.
Explanation:you could break it down to your own words
What is science? what is science?
Answers
We define science as the knowledge that seeks to understand truths or natural laws to explain .
What science concept?In the most specific sense of the word, science is that type of knowledge that seeks to understand truths or natural laws to explain the functioning of things and the universe in general.
The concept of science can be defined as a body of systematized knowledge acquired through observation, identification, research and explanation of certain categories of phenomena and facts, and formulated methodically and rationally.
See more about science at brainly.com/question/12842883
Answer:
Science is the acquisition of our own knowledge through experimentation to explain and describe natural phenomena.
Explanation:
Science comes from the Latin word, Scientia, which means knowledge. Knowledge are facts that can be verified.
characteristics. of. rusting
Answers
Answer: metal turn orange and weaker as it gets oxidised
Explanation:
pls help asap!!
The surface of earth has many impact craters that have been filled in with sediment. these craters were created by
A. moles
B. dinosaurs
C. people
D. meteorites
Answers
Answer:
D
Explanation:
Craters produced by the collision of a meteorite with the Earth (or another planet or moon) are called impact craters. The high-speed impact of a large meteorite compresses, or forces downward, a wide area of rock. The pressure pulverizes the rock.
Which of these describes a Mole in Chemistry?
A. A counting term for substances
B. The Molar mass of substance in grams
C. 6.02 x 10^23
D. All of the above describe a mole
Answers
Answer:
I think it's B, good luck
1s2 3s2 3p6 3s2 3p6 3d6 4s2 what is element
Answers
The element with the electron configuration, 1s2 3s2 3p6 3s2 3p6 3d6 4s2, will be iron.
Atomic number and electronic configurationsIn the electronic configuration of elements, the number of electrons possessed by the element is shared into the orbitals according to their energy levels.
The electrons are first shared in orbitals in the same energy level before pairing starts.
Also for neutral atoms, the number of electrons is equivalent to the number of protons. The number of protons in itself represents the atomic number of elements.
Thus, considering this electron configuration, 1s2 3s2 3p6 3s2 3p6 3d6 4s2; the total number of electrons in all the orbitals is 26. The element with the atomic number of 26 is iron.
More on electron configurations can be found here: https://brainly.com/question/14283892
#SPJ1
3. Which is larger, 5 kg or 500 g?* Easy way to remember the order of prefixes: Kids Have Dreams But Dreams Cost Money 5 kg O 5009 They are equal
what is the answers
Answers
Answer:
your mom
Explanation:
2AI + 6HCI=2AlCl3 + 3H₂
3. Aluminum reacts with HCI to produce aluminum chloride (AICI3) and hydrogen gas (H₂).
Calculate the number of moles of HCI required to react with 0.62 moles of Al.
Answers
3.0 moles of \(Al\) can fully react with hydrogen chloride to produce 4.5 moles of \(H_{2}\). Thus, 0.93 moles will be produced by 0.62 moles of \(Al\).
STOICHIOMETRYBased on this inquiry, how does aluminum react with hydrogen chloride to produce aluminum chloride and hydrogen gas\(Al +6HCl= AlCl_{3} +3H_{2}\)According to this equation, 3 moles of hydrogen gas are produced during the reaction of 2 moles of aluminum (\(Al\)).As a result, 3 moles of aluminum will result in 3 3 2 = 4.5 moles of hydrogen gas.As a result, the entire reaction of 3.0 moles of \(Al\)with hydrogen chloride can produce 4.5 moles of \(H_{2}\).The proportion of reactants to products before, during, and after chemical processes is known as stoichiometry.For more information on stoichiometry kindly visit to
https://brainly.com/question/19484482
#SPJ1
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
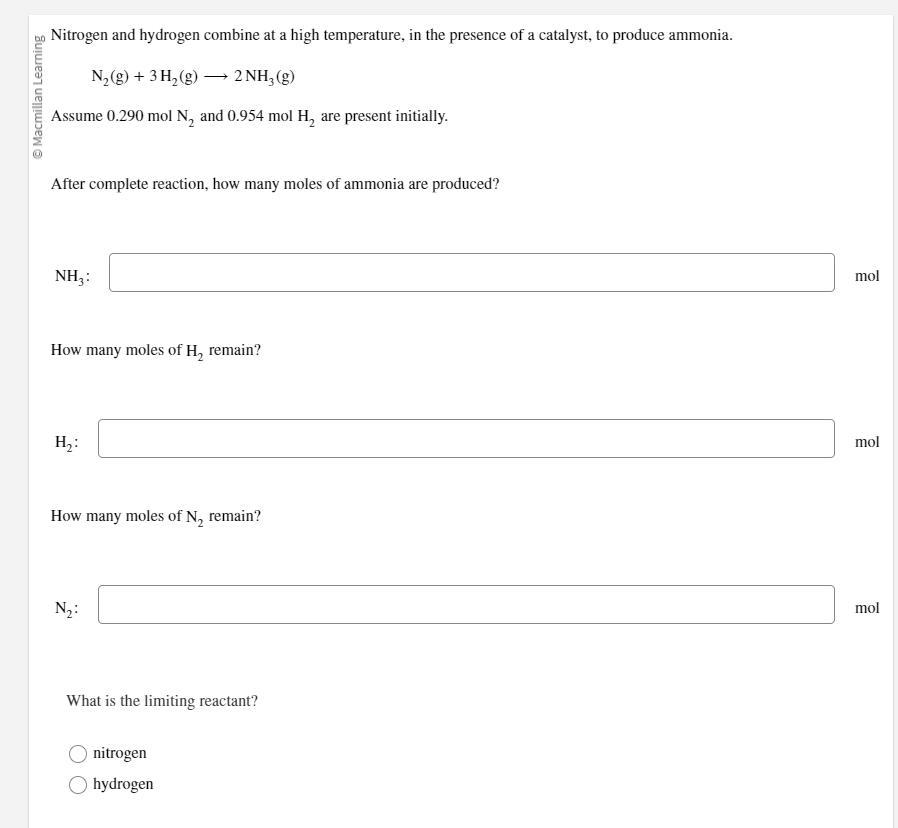
Answers
Answer:
17 reactions
Explanation:
How do scientists describe the behavior of particles in gases?
Answers
Answer:
The behavior of the particles in the gases as follows:
Particles of a gas are continually moving and colliding upon things. This gives gases their properties of pressure. The gas laws depict the connection between volume, weight, and temperature of a given measure of gas.
gas vibrate and move openly move at high speeds. In gases, the particles move quickly in every direction, often crashing into one another and the side of the gas can or container.
The size of these particles is very small compared to the difference between two neighboring particles. These particles are continually moving on the fact that they have kinetic energy that will increase with an expansion in temperature, leads to the particles to move quicker.
What is the median reaction of second end point in HCL and NaOH titration
Answers
The median reaction at the second end point in the HCl and NaOH titration is: HCl + NaOH → NaCl + H2O
In a titration between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the reaction involved is the neutralization reaction between an acid and a base. The balanced equation for this reaction is:
HCl + NaOH → NaCl + H2O
In this reaction, one mole of HCl reacts with one mole of NaOH to form one mole of NaCl (sodium chloride) and one mole of water.
During the titration process, the reaction occurs gradually as the base is added to the acid solution.
The first end point of the titration is reached when the moles of HCl and NaOH are stoichiometrically equivalent, meaning they react in a 1:1 ratio. At this point, all the HCl has been neutralized by the NaOH, and no excess of either reagent remains.
However, if the titration is continued beyond the first end point, the reaction between HCl and NaOH can still occur, albeit in a different ratio.
The second end point refers to the point where the moles of NaOH added exceed the stoichiometrically required amount to neutralize the HCl completely. As a result, any excess NaOH added after the second end point reacts with the excess HCl in a 1:1 ratio.
Therefore, the median reaction at the second end point in the HCl and NaOH titration is:
HCl + NaOH → NaCl + H2O
For more such question on median reaction visit:
https://brainly.com/question/14189499
#SPJ8
Please help!!
How many O2 molecules occupy a 1.0 L flask at 65°C and
103.7 kPa?
Select one:
O a. 28 molecules
O b. 2.2 x 1022 molecules
O c.
1.1 x 1023 molecules
O d.
1.6 x 1025 molecules
e. 1.7 x 1025 molecules

Answers
Answer:
B, 2.22×10^22 molecules
Explanation:
Given PV=nRT, n=PV/RT
n=103.7×1.0/8.314×(65+273.15)
= 0.0368858... moles
Given n= number of particles/avogadros number
number of particles=n×avogadros number
number of particles = 0.03688...×6.02x10^23
= 2.22×10^22 molecules
In the titration, 15 mL of CsOH solution is neutralized by 38.2 mL of 0.250 M HBr solution. What is the molarity of the CsOH solution?
Answers
Answer: 0.637M
10.2M
1.36M
0.0982M
Explanation: the answer is 1.36M
The molarity of the CsOH solution is 0.636 M.
What is molarity?Molarity is the concentration of any substance in a place.
The reaction is
HBr + CsOH —> CsBr + H₂O
The formula of molarity
\(M = \dfrac{n}{V}\)
\(\dfrac{0.25 \times 38.2 }{15} = 0.6366\)
Thus, the molarity of the CsOH solution is 0.636 M.
Learn more about molarity
https://brainly.com/question/2817451
#SPJ2
Hydrogen gas has a density of 0.09 , and at normal pressure and 1.01 one mole of it takes up 22.4. How would you calculate the moles in 770 of hydrogen gas?
Answers
Answer : The moles of hydrogen gas will be, 201.9 moles
Solution :
First we have to calculate the molar mass of hydrogen gas.
using ideal gas equation,
where,
n = number of moles of gas
w = mass of gas
P = pressure of the gas = 1 atm
T = temperature of the gas =
M = molar mass of hydrogen gas = ?
R = gas constant = 0.0821 Latm/moleK
D = density of gas = 0.090 g/L
Now put all the given values in the above equation, we get the molar mass of hydrogen gas.
Now we have to calculate the moles of hydrogen gas.
Therefore, the moles of hydrogen gas is, 201.9 moles
What is in period 6 group 8
Answers
Answer:
Osmium
Explanation:
If you take a look at the attached image of a periodic table below, you will see that the element in the 6th period and 8th group is Osmium. Hope this helps!

draw the structure of the product of this reaction. use the wedge/hash bond tools to indicate stereochemistry where it exists. if there are alternative structures, draw the most stable one. if no reaction occurs, draw the organic starting material.
Answers
The E2 elimination reaction of cyclo-alkylhalides are usually stereo selective. Usually trans isomer reacts slowly than cis-isomer. So the elimination reaction in an alkaline potassium hydroxide medium will be 1-methyl cyclohexene.
The reason behind stereo selectivity is because pf the spatial orientation of the substituents on the ring structure. For cyclic compounds this has a great influence to direct a reaction in Regio-selectivity. The transition state between the reactant and product influence in the rate of elimination reaction.
Here the major elimination product is 1-methyl cyclohexene and a minor amount of 3-methyl cyclohexene is also produced.
For more information regarding elimination reaction, kindly refer
https://brainly.com/question/28239179
#SPJ4
(The question is not complete. The complete question is given as an image)


Write down a balanced equation for SnO2 + H2 → Sn + H2O and tell which substance is the oxidising agent and which is the reducing agent.
Answers
Answer:
Sn is the oxidation agent and h2 is the reducing agent
Explanation:
Because oxidation agent means reduction which means the lose of oxygen and Sn lose oxygen.
While reduction agent means oxidation which also means the gain of oxygen and h2 gain oxygen.
I hope you understand my explanation if you need any help in chemistry I'm always here
Please help with c and d Will mark as brainliest!! Any help would be appreciated!

Answers
Use Q = smT to determine the metal's heat capacity. When keep in mind to account for the metal's mass, mass in heat, and change in temperature. The melting temperature of copper metal is 1083 degrees Celsius, and its specific heat is 0.385 J/g.
What is a sample's heat capacity?In thermodynamics, the massic heat capacity (also known as massic heat capacity) of a substance is indeed the heat capacity of the a sample of a substance divided by mass of the sample (symbol c).
1 joule of heat is equal to what unit?The unit of measurement for heat is the joule (J). However, for practical reasons, a unit watts (W) is commonly used since one joule per second equals one watt in heat flow. Heat flow could be expressed in terms or joules per second (J/s).
To know more about mass visit:
https://brainly.com/question/14651380
#SPJ1
What is the balanced chemical equation for wood burning
Answers
Answer:
P + O2 --> P2O5
Explanation:
For a simple match burning you can use the following equation: P + O2 --> P2O5 To balance the reaction you have to make sure you have the same amount of each element on both sides.
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
CH3-CH2-CH=CH-CH3 ( MARKOVIAN'S RULE AND ANTI MARKOVIAN'S RULE)
Answers
Answer:
Explanation:
CH3-CH2-CH=CH-CH3 is a molecule with a long carbon chain containing a double bond between the third and fourth carbon atoms. In this molecule, the first three carbon atoms (CH3-CH2-CH) form a substructure known as a propyl group, while the last two carbon atoms (CH=CH-CH3) form a substructure known as an allyl group.
When applying Markovnikov's rule to this molecule, the double bond is treated as a functional group that can react with other molecules. In this case, the double bond will react with a molecule of hydrogen to form a new carbon-hydrogen bond, with the hydrogen atom attaching to the carbon atom that already has the most hydrogens. In this molecule, the carbon atom in the propyl group has three hydrogens, while the carbon atom in the allyl group has only two hydrogens. Therefore, according to Markovnikov's rule, the hydrogen atom will react with the carbon atom in the allyl group, forming a new molecule with the following structure: CH3-CH2-CH-CH2-CH3.
Alternatively, if the reaction follows anti-Markovnikov's rule, the hydrogen atom will react with the carbon atom in the propyl group, forming a new molecule with the following structure: CH3-CH2-CH2-CH=CH3. This is the opposite of what would happen under Markovnikov's rule, as the hydrogen atom attaches to the carbon atom with the fewest hydrogens instead of the most.
Overall, Markovnikov's rule and anti-Markovnikov's rule describe how double bonds in molecules can react with other molecules to form new bonds. These rules help predict the outcome of chemical reactions involving double bonds, and are important tools in organic chemistry.
What is the volume of 1g of ice in cm3?
Answers
Answer:
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
What is the volume of 1g of ice in cm3?
Explanation:
Classify each of the picture below by placing the correct label in the blanks below. SOMEONE PLEASE HELP ME

Answers
The classification of the substances are as follows;
1) C
2) MOE
3) MOEAC
4) MOC
5) E
6) C
7) E
8) MOEAC
9) MOE
10) MOEAC
11) C
12) MOC
13) MOC
14) MOC
15) MOC
What are compounds, mixtures and elements?An element is a pure substance that cannot be broken down into simpler substances by chemical means. Elements are composed of only one type of atom, and they are listed on the periodic table. For example, oxygen, gold, and carbon are all elements.
A compound is a substance composed of two or more elements chemically combined in a fixed ratio. The properties of a compound are different from those of the elements that make it up. For example, water is a compound made up of two elements, hydrogen and oxygen, and has different properties than its constituent elements.
A mixture is a combination of two or more substances that are not chemically combined. Mixtures can be composed of elements, compounds, or both, and they can be separated by physical means.
Learn more about compound:https://brainly.com/question/13516179
#SPJ1
if the body is moving with uniform acceleration then, eng of motion are given as s = u+v/2+t
Answers
Yes, s = u+v/2+t, where s is the displacement, u is the beginning velocity, v is the end velocity, and t is the time required, is the equation of motion for a body travelling with uniform acceleration.
The basic law of motion, which states that a body's rate of change in displacement is directly proportional to its velocity, provides the basis for this equation. The equation of motion for a body travelling with constant acceleration, s = ut + 1/2at2, may be used to derive it.
The equation of motion for a body travelling with uniform acceleration is given by replacing the value of an as (v-u)/t and getting s = u+v/2+t. This formula is only accurate when the body's acceleration is constant and uniform.
Learn more about acceleration at:
https://brainly.com/question/12550364
#SPJ1