In this laboratory, two methods are used to determine the identity of unknown cations,
the qualitative scheme and flame test. What is the primary advantage of the qualitative
scheme compared to the flame test?
O The qualitative scheme is less complicated to implement
The qualitative scheme is faster to perform
The qualitative scheme is more sensitive
O The qualitative scheme is able to distinguish mixtures of unknowns
Answers
The qualitative method of analysis of cations depends on chemical reactions. It has the advantage of being able to distinguish mixtures of unknowns.
The flame test you can only detect a single cation with precision. If there is a mixture of cations, you can only detect the cation that burns with the brightest flame in the mixture. This makes the flame test limited in its applications.
However, the qualitative scheme is useful in distinguishing a mixture of unknown cations because each cation undergoes a remarkable chemical reaction with certain reagents.
Learn more: https://brainly.com/question/6955504
Related Questions
The strongest bases have pH values close to
Answers
Answer:
Explanyour mmation:
Atomic structure and bonding
Answers
Hi! Formula:- Physics of Nondestructive Evaluation > Materials and Processes > Atomic Bonding
Atomic Bonding
In the Bohr model, the nucleus is composed of protons and neutrons. Atomic number identifies the number of protons in the nucleus. Atomic mass is the sum of protons and neutrons. Electrons orbit the nucleus. Protons and electrons have equal but opposite electrical charge.From elementary chemistry it is known that the atomic structure of any element is made up of a positively charged nucleus surrounded by electrons revolving around it. An element’s atomic number indicates the number of positively charged protons in the nucleus. The atomic weight of an atom indicates how many protons and neutrons in the nucleus. To determine the number of neutrons in an atom, the atomic number is simply subtracted from the atomic weight.
Atoms like to have a balanced electrical charge. Therefore, they usually have negatively charged electrons surrounding the nucleus in numbers equal to the number of protons. It is also known that electrons are present with different energies and it is convenient to consider these electrons surrounding the nucleus in energy “shells.” For example, magnesium, with an atomic number of 12, has two electrons in the inner shell, eight in the second shell and two in the outer shell.
All chemical bonds involve electrons. Atoms will stay close together if they have a shared interest in one or more electrons. Atoms are at their most stable when they have no partially-filled electron shells. If an atom has only a few electrons in a shell, it will tend to lose them to empty the shell. These elements
are metals. When metal atoms bond, a metallic bond occurs. When an atom has a nearly full electron shell, it will try to find electrons from another atom so that it can fill its outer shell. These elements
are usually described as nonmetals. The bond between two nonmetal atoms is usually a covalent bond. Where metal and nonmetal atom come together an ionic bond occurs. There are also other, less common, types of bond but the details are beyond the scope of this material.
Here is a picture

make lead reactivity equation
Answers
The reactivity of lead is relatively low compared to other metals, but it is still an important element in many industrial and chemical processes.
To make a lead reactivity equation, we need to first understand the chemical properties of lead. Lead is a heavy metal with the chemical symbol Pb and atomic number 82. It is a highly reactive element and can easily form compounds with other elements.
The most common reaction involving lead is its reaction with hydrochloric acid (HCl). The balanced equation for this reaction is:
Pb + 2HCl → PbCl2 + H2
This reaction involves lead reacting with hydrochloric acid to produce lead chloride and hydrogen gas. Lead chloride is a white solid that is insoluble in water.
Another common reaction involving lead is its reaction with sulfuric acid (H2SO4). The balanced equation for this reaction is:
Pb + H2SO4 → PbSO4 + H2
This reaction involves lead reacting with sulfuric acid to produce lead sulfate and hydrogen gas. Lead sulfate is a white solid that is insoluble in water.
In addition to these reactions, lead can also react with oxygen to form lead oxide (PbO) and with carbon dioxide to form lead carbonate (PbCO3).
Overall, the reactivity of lead is relatively low compared to other metals, but it is still an important element in many industrial and chemical processes.
For more such questions on reactivity , Visit:
https://brainly.com/question/25103661
#SPJ11
How many bonding electrons are in the Lewis structure of PCl₃?
5
6
4
2
Answers
Answer:
B.) 6
Explanation:
Attached below is the Lewis structure of PCl₃ . Since phosphorus (P) has 5 valence electrons and chlorine (Cl) has 7 valence electrons, there should be 26 valence electrons (5 + 7(3) = 26) in the Lewis structure.
Bonding electrons are the electrons present in the chemical bonds between two atoms.
There are 2 electrons shared in every single bond. Within PCl₃, there are 3 single bonds. As such, there are 6 bonding electrons in the Lewis structure of PCl₃.

what is the concentration of a nitric acid solution if 10.0 ml of the solution is neutralized by 3.6 ml of 0.2 m naoh?
Answers
Answer:
The concentration of the nitric acid (HNO3) solution is 72 M.
Explanation:
To determine the concentration of the nitric acid solution, we can use the concept of stoichiometry and the equation of the neutralization reaction between nitric acid (HNO3) and sodium hydroxide (NaOH):
HNO3 + NaOH → NaNO3 + H2O
The balanced equation shows that the molar ratio between HNO3 and NaOH is 1:1. This means that 1 mole of HNO3 reacts with 1 mole of NaOH.
Given:
Volume of HNO3 solution = 10.0 ml
Volume of NaOH solution = 3.6 ml
Molarity of NaOH solution = 0.2 M
To find the concentration of the HNO3 solution, we need to calculate the number of moles of NaOH used in the neutralization reaction:
moles of NaOH = volume of NaOH solution * molarity of NaOH solution
= 3.6 ml * 0.2 M
= 0.72 mmol (millimoles)
Since the molar ratio between HNO3 and NaOH is 1:1, the number of moles of HNO3 in the solution is also 0.72 mmol.
Now, we can calculate the concentration of the HNO3 solution using the formula:
concentration (in M) = moles of solute / volume of solution (in L)
concentration = 0.72 mmol / 0.010 L
= 72 mmol/L
= 72 M
Therefore, the concentration of the nitric acid (HNO3) solution is 72 M.
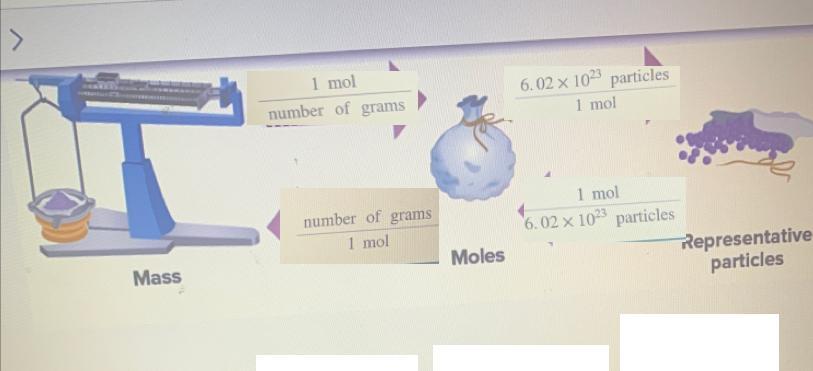
Drag each conversion factor to the arrow that indicates where it should be used

Answers
Explanation:
mass ----> moles ---> representative particles:
To go from mass to moles we usually use the molar mass of the substance or compound that we are working with. Molar mass is expressed in g/mol, so we divide by the molar mass.
mass --> moles: g * 1 mol/(number of g) = mol
To go from moles to representative particles we use Avogadro's number. There are 6.02 *10^23 particles in 1 mol of particles.
moles ---> particles: moles * 6.02 * 10^23 particles /(1 mol) = particles
Then to go from particles to moles we do something similar.
particles---->moles: particles * 1 mol/(6.02 * 10^23 particles) = moles
And to go from moles to grams, instead of dividing by the molar mass we multiply by it.
moles --> mass: moles * number of g/(1 mol) = g
Answer:
mass ----> 1 mol/(number of g) ---> moles ---> 6.02 * 10^23 particles /(1 mol) ----->particles
mass <---number of g/(1 mol) <----- moles <----1 mol/(6.02 * 10^23 particles)<----- particles
What amount of heat (in kJ) is required to convert 13.7 g of an unknown liquid (MM = 83.21 g/mol) at 19.2 °C to a gas at 93.5 °C? (specific heat capacity of liquid = 1.58 J/g・ °C; specific heat capacity of gas = 0.932 J/g・ °C; ∆Hvap = 22.5 kJ/mol; normal boiling point, Tb = 57.3 °C)
Answers
Give the hybridization for the C in HCN. Give the hybridization for the C in HCN. sp3d2 sp3 sp2 sp sp3d
Answers
Answer:
The correct answer is - sp hybridization.
Explanation:
The molecular structure line with a carbon bound to the hydrogen with the single sigma bond and with 2 pi bond and one single sigma bond with carbon Nitrogen triple covalent bond.
To calculate the hybridization of an element like carbon by adding the sigma bond and the lone pair of that atom as here in HCN molecule, zero lone electrons and two sigmas bond the C atom includes sp-hybridized orbital.
The hybridization for the carbon (C) atom in HCN (hydrogen cyanide) is sp. Therefore, option D is correct.
The electron configuration of carbon is 1s² 2s² 2p², with four valence electrons. In HCN, carbon forms three sigma bonds: one with hydrogen (H) and two with nitrogen (N). The sigma bonds are formed by overlapping atomic orbitals.
In the process of hybridization, the carbon atom promotes one of its 2s electrons to a 2p orbital to create a set of four hybrid orbitals. In HCN, only three hybrid orbitals are involved in bonding. One sp hybrid orbital of carbon overlaps with the 1s orbital of hydrogen to form the C-H sigma bond. The other two sp hybrid orbitals of carbon overlap with two nitrogen atoms, forming the C-N sigma bonds.
Learn more about hybridization, here:
https://brainly.com/question/29020053
#SPJ6
ss of the
ider
the
s with
ere a
plume
Sample
Oxygen 1 (0₂)
Oxygen 2 (0₂)
Hydrogen (H₂)
Volume (L)
22.41
44.8L
22.41
Number of
moles
1
2
1
HINT: Calculating Mass
Return to Samples
Molar Mass
(g/mol)
Mass of gas
sample (g)

Answers
Molar mass of 0₂= 16 and H₂= 2 g.
Molar mass is defined as the mass per mole. In other words, molar mass is the sum of the masses of all the atoms that make up a mole of a material. It is expressed in values of grams per mole.
The molar mass of the elements or molecules is shown. In the case of single elements or individual atoms, the molar mass is just the element's mass given in atomic mass units. In other words, the atomic mass and molar mass of an atom are same. Because mol mass is comparable to atomic mass for individual atoms, it may be used to identify a particle.
To know more about molar mass visit : https://brainly.com/question/13128557
#SPJ9
( this is not a question)
Drag the tiles to the correct boxes to complete the pairs.
A property of a substance can be measured or observed. Match each attribute of the substance with the property that describes it.

Answers
Answer:
They are alla correct
Explanation:
Good morning, good afternoon, and good evening
(IF u understand this reference u are a god)
Answer
Hi!
1. Why does you mouth close when you say
"open" and opens when you say "close"?
2. When you come out of a shower clean
how does the towel get dirty?
Explanation:
Cause god is built different
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
describe ways to seperate heterogeneous mixtures
Answers
Sieving is typically used to separate particles by size through a mesh, like what you see near construction sites.
Filtration is a method used to separate insoluble solid from liquids using filter paper.
Handpicking is a method used to separate a mixture using hands.
There’s also distillation which is a process to separate components of a homogeneous mixture. It involves heat and a sophisticated apparatus, which is why it’s not considered a simple method.
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
3 The chemical compositions of two substances, W and X, are given.
W Na(AISI³)O8
X Ca(Al₂Si₂)08
Which statements are correct?
1 W and X contain the same amount of oxygen.
2 W contains three times as much silicon as X.
3 X contains twice as much aluminium as W.
A) 1 and 2 B) 1 and 3 C) 2 and 3 D) 1, 2 and 3
Answers
Answer:
D) 1,2 and 3 is the answer
Determine the number of moles of sodium carbonate (Na2CO3) in a 0.2120 g sample. This sample of Na2CO3 was used to standardise a stock solution of hydrochloric acid in which the Na2CO3 was fully neutralised. If 10.52 cm3 of the HCl solution was required determine the concentration of the HCl in mol dm–3.
Answers
The number of moles of the sodium carbonate would be 0.00200 mol.
The concentration of the HCl would be 0.190 mol/dm^3.
Number of molesTo determine the number of moles of sodium carbonate (Na2CO3) in the given sample, we first need to calculate its molar mass:
Na2CO3 molar mass = 2(22.99 g/mol) + 12.01 g/mol + 3(16.00 g/mol) = 105.99 g/mol
moles of Na2CO3 = mass of sample / molar mass
moles of Na2CO3 = 0.2120 g / 105.99 g/mol
moles of Na2CO3 = 0.00200 mol
Now, to determine the concentration of the hydrochloric acid (HCl) solution, we can use the following equation:
moles of Na2CO3 = moles of HCl
We know that 10.52 cm3 of the HCl solution was required to fully neutralize the Na2CO3. Let's assume that the concentration of the HCl solution is x mol/dm^3. Then we can use the following equation:
moles of HCl = concentration of HCl x volume of HCl solution (in dm3)
0.00200 mol = x mol/dm^3 x 0.01052 dm^3
Solving for x, we get:
x = 0.00200 mol / 0.01052 dm^3 = 0.190 mol/dm^3
Therefore, the concentration of the hydrochloric acid solution is 0.190 mol/dm^3.
More on number of moles can be found here: https://brainly.com/question/12513822
#SPJ1
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
what is the composition of lassaigne extract in organic compound containing nitrogen? why is it alkaline in nature? how is presence of nitrogen detected?
Answers
Answer:
Di ko po alam eyan ehh sorry po uWu
Explanation:
Sorry
When writing a lab report where do your data tables belong
A. Conclusion
B. Materials
C. Purpose
D. Results
Answers
Answer:
Results or D.
Explanation:
I think it’s d. or results because normally that’s were I would put my information once I have my experiment done.
What is used to determine the number of each atom in an ionic formula?
A. The oxidation state of each atom
B. The number of bonds in the formula
C. The electronegativity of each atom
D. The period of the metal in the formula
Answers
the oxidation state of each atom
120cm3 of a gas at 25°c exerts a pressure of 750mmHg. calculate its pressure if its volume increased to 150cm3 at 40°c.
Answers
Answer:
P2 = 1125 mmHg
Explanation:
Gas Pressure Calculation
To solve this problem, we need to use the combined gas law, which relates the pressure, volume, and temperature of a gas under different conditions. The combined gas law is given by:
(P1V1)/T1 = (P2V2)/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature of the gas, and P2, V2, and T2 are the final pressure, volume, and temperature.
Let's start by calculating the initial conditions:
P1 = 750 mmHg
V1 = 120 cm^3
T1 = 25°C + 273.15 = 298.15 K (temperature in Kelvin)
Now we can plug in these values and solve for P2:
(P1V1)/T1 = (P2V2)/T2
(750 mmHg x 120 cm^3) / 298.15 K = (P2 x 150 cm^3) / (40°C + 273.15)
Simplifying this equation, we get:
P2 = (750 mmHg x 120 cm^3 x (40°C + 273.15)) / (298.15 K x 150 cm^3)
P2 = 1125 mmHg
Therefore, the pressure of the gas would increase to 1125 mmHg if its volume increased to 150 cm^3 at 40°C.
ChatGPT
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
How many yards are in a 100 meter race ? How many feet?
Answers
Taking into account the change of units, 100 meters are 109.361 yards and 328.084 feets.
Rule of threeThe rule of three is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them.
That is, what is intended with it is to find the fourth term of a proportion knowing the other three.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied.
To solve a direct rule of three, the following formula must be followed, being a, b and c known data and x the variable to be calculated:
a ⇒ b
c ⇒ x
So: \(x=\frac{cxb}{a}\)
The direct rule of three is the rule applied in this case where there is a change of units.
Distance in yards and feetTo perform in this case the conversion of units, you must first know that:
1 m= 1.09361 yards1 m= 3.28084 feetsThen, you can apply the following rules of three:
if 1.09361 yards is 1 meter, how many yards equals 100 meters?1 meter ⇒ 1.09361 yards
100 meters ⇒ x
So: \(x=\frac{100 metersx1.09361 yards}{1 meter}\)
Solving:
x= 109.361 yards
if 3.28084 feets is 1 meter, how many yards equals 100 meters?1 meter ⇒ 3.28084 feets
100 meters ⇒ x
So: \(x=\frac{100 metersx3.28084 feets}{1 meter}\)
Solving:
x= 328.084 feets
In summary, 100 meters are 109.361 yards and 328.084 feets.
Learn more about rule of three and change of units with this example:
brainly.com/question/12482948
#SPJ1
Calibrating the table top balance at 0, 50 and 100 grams is called

Answers
Calibrating the tabletop balance at 0, 50, and 100 grams is called linear Calibration.
In easy phrases, calibration is a quantitative contrast. to test the reading of stability or scale, a reference weight is located at the pan. the mistake is defined because of the distinction between the measured fee (the analyzing) and the true cost the reference weight.
The primary importance of calibration is that it keeps accuracy, standardization, and repeatability in measurements, assuring reliable benchmarks and results. without everyday calibration, the device can fall out of spec, offer inaccurate measurements and threaten first-class, safety, and device longevity.
Calibration is vital as it allows ensures accurate measurements, and accurate measurements are foundational to the excellent, protection and innovation of maximum products and services we use and rely upon each day.
Learn more about Calibrating here:-https://brainly.com/question/787793
#SPJ9
Gold has a latent heat fusion of 64.5 J/G. How much energy is required to melt 20.0 G of gold
Answers
Answer:
1290 Joules
Explanation:
Cho biết độ tan của NH4Cl trong nước ở 20oC và 70oC lần lượt là 37,2 g/100 gam nước và 60,2 gam/100 g nước. Hòa tan 166,8 gam NH4Cl vào 400 gam nước ở 70oC thu được dung dịch X. Sau đó, hạ nhiệt độ dung dịch X xuống 20oC. Tính khối lượng (gam) NH4Cl kết tinh lại trong X?
Answers
Answer: Hợp chất CTHH 0 °C 10 °C 20 °C 30 °C 40 °C 50 °C 70 °C
Actini(III) hydroxide Ac(OH)3 0,0022
Amonia NH3 1176 900 702 565 428 333 188
Amoni azua NH4N3 16 25,3 37,1
View 42 more rows
hehe
7. The image shows the effects of exposing the plants to different color of
lights. The size of the plant was measured and the number of leaves were
counted if they are alive or dead. Size of plants and number of leaves are
Size of Plant
Number of Leaves
Living or Dead?
science or
What’s the answer?
Answers
Answer:
An apple, potato, and onion all taste the same if you eat them with your nose plugged
Explanation:
I need help ASAP please giving brainliest!!

Answers
Explanation:
im bumb like really bumb
Which Example is a way that intercellular communication occurs
A. A molecule is sent from one part of a cell to another
B. A signal is sent from a nerve cell to a muscle cell
C. A signal is sent down an exon within a nerve cell
D. A signal is translated into action for a muscle cell to contract
Answers
Answer:
B) A signal is sent from a nerve cell to a muscle cell.
Explanation:
The signal, an impulse called an action potential, travels through a type of nerve cell called a motor neuron. The neuromuscular junction is the name of the place where the motor neuron reaches a muscle cell. Skeletal muscle tissue is composed of cells called muscle fibers.
The correct answer is:
B.(A signal is sent from a nerve cell to a muscle cell)
I took the test, hope this helps!

WILL MARK BRAINLIEST
What occurs when energy is added or removed from an object or a system by an outside agent?
Potential Energy
Kinetic Energy
Elastic Potential Energy
Thermal Energy
Work
Answers
Answer:
Its Thermal Energy
Explanation: