In the electrolysis of molten NaBr,(a) What product forms at the anode?
Answers
\(2 Br^- (aq)\) -----> \(Br_2 (g)\) + \(2e^{-}\)
The product formed at the anode is \(Br_{2}\) (g).
Bromine can be produced by the electrolysis of molten sodium bromide.
In molten state ⇒ ions can move freely
Anions move towards the anode (oxidation site)
Cations move towards the cathode (reduction site)
So electrolysis extract electron from \(Br^-\) and force electron onto \(Na^+\)
In chemistry, the type of process in which decomposition of a chemical substance occurs due to the flow of electricity through the substance is generally known as the electrolysis process. Usually, the electrolysis process becomes in an arrangement in which one electrode of the cathode and one electrode of the anode are present.
In chemistry and production, electrolysis is a technique that makes use of direct electric present-day (DC) to power an otherwise non-spontaneous chemical response.
Electrolysis is commercially crucial as a degree inside the separation of factors from clearly going on sources including ores the usage of an electrolytic cell.
To learn more about the electrolysis please click on the link : https://brainly.com/question/24063038
#SPJ4
Related Questions
can someone help me please
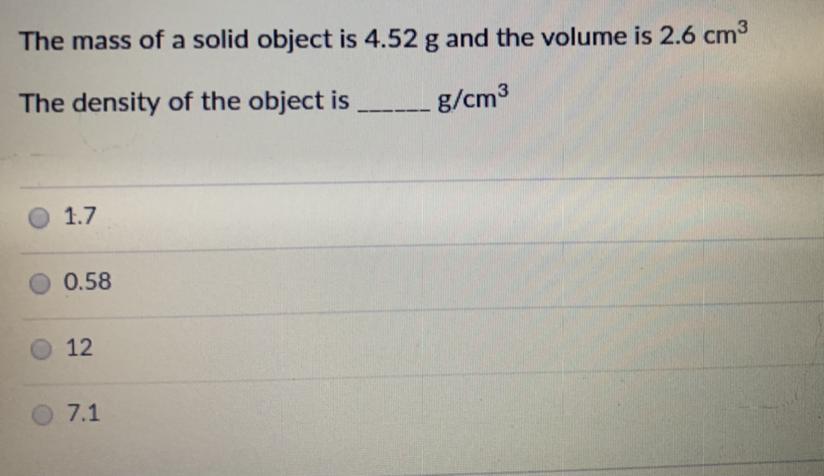
Answers
10. How many moles of CuCl, are in 150 L of a 2.33 M Cuci, solution?
Answers
Answer:
349.5 moles
Explanation:
(I am assuming that there is a typo at the end, where Cuci solution = CuCl solution)
The formula for molarity is:
Molarity = moles in solution / volume of solution
Here, we are given both the Molarity and the volume. So we can plug it into the equation above and get:
2.33 = moles / 150
Now we solve for the moles by doing:
moles = 2.33 * 150
This gives us the answer of 349.5 moles.
please help. I was hoping someone could explain it , cause I have no idea what I'm doing PLEASE HELP. ASAP!!!!

Answers
The additional information that is required to obtain the enthalpy of neutralization is the density of the solution.
What is the enthalpy?We know that the enthalpy of neutralization is defined as the heat that is evolved when an acid is neutralized by a base under standard conditions. We can be able to obtain the enthalpy of the neutralization of the acid and the base by performance of some simple chemical calculations.
To carry out this calculations we need the all of the pieces of information that have been listed in the question such as the masses of the solution, the temperature of the solution in the initial and final states and so on.
Learn more about enthalpy:https://brainly.com/question/7827769
#SPJ1
a 13.597 g sample of a compound that contains sulfur is analyzed by precipitating all the sulfur as baso4. if 11.095 g of baso4 are obtained in the analysis, what is the percentage of sulfur in the original compound?
Answers
12.308% of the chemical is sulfur. The compound's whole sulfur content is transformed into barium sulfate. Therefore, the mass for sulfur from barium sulfate will match the mass for sulfur in the compound.
Is sulfur harmful to people?People are not highly poisonous to sulfur. However, consuming far to much sulfur may result in diarrhea or a burning feeling. Sulfur dust inhalation might irritate the airway or make one cough. The eyes and skin may also become irritated by it.
Where can you find sulfur?Sulfur can indeed be found in sulfide ores and in nature. It is native to areas close to volcanoes and hot springs. The tenth most common element, sulfur, is found in almost all types of plant and animal life, meteorites, ocean ocean, the humankind's crust, and or the atmosphere.
To know more about sulfur visit:
https://brainly.com/question/13469437
#SPJ4
different between larva of a silk work and larva of a bee
Answers
A mixture has a mas of 450 grams. Of that mixture,35 grams is sulfur . What is the percentage of sulfur in the mixture
Answers
A mixture has a mas of 450 grams. Of that mixture,35 grams is sulfur . Therefore, 11% is the percentage of sulfur in the mixture.
What is mass percentage?The mass percent equation is defined as the mass of each element in one mole of the compound, as well as the molar mass. With these masses, you can calculate each element's mass proportion.
In order to represent the mass percent of a solution, the kilograms of solute are divided by the kilograms of solution, and the result is multiplied by 100.
percentage of sulfur = mass of sulfur/ mass of mixture×100
mass of sulfur= 5 grams
mass of mixture =450 grams
substituting all the given values in the above equation, we get
percentage of sulfur = 5 / 450=0.011×100= 11%
Therefore, 11% is the percentage of sulfur in the mixture.
To know more about mass percentage, here:
https://brainly.com/question/28973847
#SPJ1
given 1kg= 2.20 lbs, how many kilograms are in 338 kg
Answers
Answer:
154
Explanation:
Which property explain the trend in volatility of the element going down group 17
Answers
Due to an increase in the number of electrons, Group 17 elements become less volatile as they move down the group.
What is element and explain?Simply put, an element is any substance that cannot be converted into another substance. Each of the approximately 150 elements has a unique form of atom. Atoms from at least two atom, and maybe more, are present in every item in the cosmos. Every known element is listed in the periodic table, and related elements are grouped together.
What exactly is an element in chemistry?A fundamental object that is difficult to divide into smaller bits is known as an ingredient. An element is anything that cannot be broken by quasi reactions in chemistry and physics. One of humanity's most beneficial components to humanity is silicon.
To know more about Element visit:
https://brainly.com/question/14347616
#SPJ4
Sugar is C6H12O11 and ammonia is NH3 . If you have 7 moles of both Sugar and ammonia do you have the same amount
Answers
This problem is providing us with the moles of both sugar and ammonia and asks for a comparison between the moles and their amounts in grams. At the end, one concludes they have different amounts as 1,743.42 g and 119.21 g.
Mole-mass relationshipsIn chemistry, mole-mass relationships are used to calculate grams from moles or vice versa, according to specific conditions. In such a way, for sugar and ammonia, one can first calculate their molar masses with:
\(MM_{C_6H_{12}O_{11}}=6*12.01+12*1.01+11*16.00=249.06g/mol\\ \\MM_{NH_3}=14.01+1.01*3=17.03g/mol\)
Next, we calculate grams with the following proportional factors:
\(7molC_6H_{12}O_{11}*\frac{249.06gC_6H_{12}O_{11}}{1molC_6H_{12}O_{11}} =1743.42g\\\\7molNH_3*\frac{17.03gNH_3}{1molNH_3}=119.21gNH_3\)
Thus, we conclude that despite having equal number of moles, they differ in the amount of grams because of their dissimilar molar masses.
Learn more about mass and moles: https://brainly.com/question/26727229
1. Ozone in Earth’s atmosphere is important because it
A)absorbs harmful pollution.
B)absorbs harmful radiation.
C)helps clouds form.
D)causes rain to fall.
2.
A. a
B. b
C. c
D. d

Answers
Answer:
B
Explanation:
This is the process that cells reproduce and replace old or damaged cells.
Question 5 options:
Mitosis
Meiosis
Ribosomes
Cytokinesis
Answers
Answer:
Mitosis
Explanation:
This is the process that cells reproduce and replace old or damaged cells.
Calculate the wavelength of light if the energy is 3.82 x 10^-18 J?
Answers
Answer:
hi hehe I'm new here hehehe
There are three major parts within each dna nucleotide. of these three, which is responsible for the storage of genetic information?
Answers
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to
purify chemical substances. The pressures used in this procedure range from around 500
kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know
the pressure in torr. If an HPLC procedure is running at a pressure of 3.20x10^8 Pa , what is its
running pressure in torr?
Express the pressure numerically in torr.
►
3.20 x 10^8 Pa = _____ torr

Answers
Answer:
2,400,000 torr (3 s.f.)
Explanation:
Convert the pressure from Pascal to atm first:
\(\boxed{1 \: atm = 101325 \:Pa }\)
3.20 ×10⁸ Pa
= [(3.20 ×10⁸) ÷101325] atm
= 3158.2 atm (5 s.f.)
Convert atm to torr:
\(\boxed{1 \: atm = 760 \: torr}\)
3158.2 atm
= (3158.2 ×760) torr
= 2400000 torr (3 s.f.)
When energy changes from one form to another (for example, chemica
energy - heat — light), it is know as:
Select one:
energy transfer
fossil fuel chang
light heat change
energy transformation
Answers
Answer:
energy transformation
Explanation:
energy transformation is when energy changes from one form to another. like in a hydroelectric dam that transforms the kinetic energy of water into electrical energy.
Jackson E&M:
Find the interaction energy between the dipoles in the following configuration. The distance between the dipoles is "a" in all of the cases:
Answers
The interaction energy between the dipoles in the given configuration is 1/2 * k * p1 * p2 * (1/r^3) * (3cos^2(theta) - 1) r is the distance between the dipoles.
Given, the distance between the dipoles = a. The formula to calculate the interaction energy between two dipoles is given by, Interaction energy between two dipoles = (1/4πε) [(p1.p2 - 3(p1.r)(p2.r)/r^3]Where, p1 and p2 are magnitudes of the dipoles, r is the distance between the dipoles, and ε is the permittivity of free space.
Interaction energy between the dipoles = (1/4πε) [(p1.p2 - 3(p1.r)(p2.r)/r^3]On substituting the values in the above formula, we get, Interaction energy between the dipoles = 1/2 * k * p1 * p2 * (1/r^3) * (3cos^2(theta) - 1)where k is Coulomb's constant, p1 and p2 are the magnitudes of the dipoles, r is the distance between the dipoles, and theta is the angle between the dipole moments.
To know more about dipoles visit:
https://brainly.com/question/30762865
#SPJ11
Find the molecular weight of CO
Answers
Answer:
The molecular weight of CO is 28.01g/mole.
K-42 has a half-life of 12.0 hours. At present, a given ore sample contains 34.2 mg of K-42. How much did it contain yesterday at the same time?
Answers
The amount of sample of K-42 yesterday at the same time is equal to 136.8 mg.
What is the half-life of radioactive material?The half-life of the radioactive material can be described as the time required to decrease its original amount to half after decay. The half-life of a radioactive element does not depend on the initial amount of the substance.
Consider that N₀ is the initial amount of the K-42 sample
Consider that 'n' is the number of half-lives of K-42.
The time for which K-42 decay, t = 24 hrs
Half-life of substance (t½) = 12 hrs
t = n ×t½
24 = n × 12
n = 2
Assume that N₀ is the amount of the K-42 before decay for 24 hours.
N ×2² = N₀
34.2 × 4 = N₀
N₀ = 136.8 mg
Learn more about half-life period, here:
brainly.com/question/14521252
#SPJ1
if 25 cm3 of potassium hydroxide is neutralised by 20 cm 3 of 2.5 mol dm- sulfuric acid. what is the molarity of the alkali?
Answers
The molarity of the alkali potassium hydroxide is 4.167 M.
Molarity (M) is the amount of a substance in a sure quantity of answers. Molarity is described because the moles of a solute according to liters of an answer. Molarity is also called the molar attention of an answer.
Calculation:-
V = 24 cm³ = 0.024 L
V = 20 cm³ = 0.020 L
molarity of sulphuric acid = 2.5
Mv = MV
M 0.024 = 0.02 × 2.5 × 2
M = 0.1 /0.024
= 4.167 M
Molar concentration is a measure of the attention of a chemical species, specifically of a solute in an answer, in terms of the amount of substance in step with the unit extent of the answer. In chemistry, the most generally used unit for molarity is the number of moles in keeping with liter, having the unit symbol mol/L or mol/dm³ in SI unit.
Learn more about molarity here:-https://brainly.com/question/14469428
#SPJ1
what do you think happens to a sound wave when the volume of sound increase
Answers
As the volume of the sound is seen to increase, the sound that we hear is louder as the amplitude of the sound increases.
What is sound?Sound is a kind of wave that moves through a medium. We know that sound has to move via several compressions and rare factions. This implies that sound is as well propagated through air. The movement of the sound waves through air is because the air is set into vibration by the sound. Thus, the air is very imprtant in the movement of the sound waves.
With this much said, we can see that if there is more air, there would more molecules that can be set into vibration and the sound wave would tend to increase and that is the deal that we are trying to communicate here.
Thus, the amplitude of air increases as the volume of the sound gets higher and the sound that we hear is much louder.
Learn more about sound waves:https://brainly.com/question/21995826
#SPJ1
I need help plssss
4.01

Answers
The layers of the atmosphere are;
Exosphere - A
Thermosphere - E
Mesosphere - B
Stratosphere - E
Troposphere - C
What are the layers in the atmosphere?The troposphere, which is the lowest part of the atmosphere, rises from the surface of the Earth to an average height of roughly 18 kilometers (12 miles) at the equator and 12 kilometers (7.5 miles) in the poles. The majority of the Earth's air mass is present there, making it the location of weather occurrences. In the troposphere, temperature typically drops as altitude rises.
The thermosphere is located above the mesosphere and stretches from a height of around 85 kilometers (53 miles) up to 600 kilometers (370 miles). Due to the incredibly low molecular density in the thermosphere, despite the high temperatures that are there, it does not feel warm. The auroras also appear in this layer.
Learn more about atmosphere:https://brainly.com/question/32274037
#SPJ1
What type of relationship exists between
dissociation constant and pk values
A Linear
B Directly Proportional
C Direct
D Inverse
Answers
Answer: D inverse . The product of Ka and Kb equals the auto-dissociation constant for water.
Explanation: i hoped that helped and Plz give as a brainliest !!
Calculate the number of moles in 12.50 g of benzene C2H5OH.
Answers
The number of moles of Benzyl alcohol is 0.115 moles.
Number of moles can be also told in terms of Avogadro's number with is 6.023 × \(10^{23}\). It can be calculated for atoms, molecules, ions or others as well. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance. The concept of the mole helps to put quantitative information about what happens in a chemical equation on a macroscopic level. So it is used widely.
Molecular mass is nothing but the sum of atomic mass of all the element present in the molecule. In the above given question we have to calculate the number of moles of the molecule by using the given weight. To do so we use the below formula;
Number of moles = given weight
Molecular mass
⇒ 12.50 = 0.115 moles
108.14
Therefore the number of moles in 12.50 g of benzene C2H5OH is 0.115 moles
To know more about number of moles
https://brainly.com/question/105061
#SPJ1
If H 2
and Cl 2
are mixed, how will they interact based on your knowledge of chemical bonds?
Answers
The reaction of the hydrogen and the chlorine atoms would lead to the formation of HCl
Formation of HClIn this reaction, each chlorine molecule (Cl2) breaks apart into two chlorine atoms (Cl), and each hydrogen molecule (H2) breaks apart into two hydrogen atoms (H). These reactive atoms then combine to form hydrogen chloride molecules (HCl).
It's worth noting that the reaction between hydrogen gas and chlorine gas is highly exothermic and releases a significant amount of energy.
Learn more about HCl:https://brainly.com/question/30233723
#SPJ4
which one of the following conditions is always true for a titration of a weak acid with a strong base? the equivalence point occurs at a ph equal to 7. if a colored indicator is used, it must change color rapidly in the weak acid's buffer region. a colored indicator with a pka less than 7 should be used. equal volumes of weak acid and strong base are required to reach the equivalence point. the equivalence point occurs at a ph greater than 7.
Answers
The correct option is D, The equivalence point occurs at a pH greater than 7 for a titration of a weak acid with a strong base. This is because the strong base will react with the weak acid to form a salt and water.
Titration is a commonly used analytical technique in chemistry for determining the concentration of an unknown solution by adding a known amount of a standardized solution of known concentration. The process involves slowly adding the standardized solution to the unknown solution until the chemical reaction between the two is complete. The point at which the reaction is complete is known as the equivalence point and can be detected using various indicators that change color or other properties at this point.
The main aim of titration is to accurately measure the concentration of a particular substance in a solution. For example, an acid-base titration can be used to determine the concentration of an acid in a solution by adding a known amount of a strong base until the equivalence point is reached. Similarly, a redox titration can be used to determine the concentration of a reducing or oxidizing agent in a solution.
To know more about Titration refer to-
brainly.com/question/31483031
#SPJ4
Given the mass number and atomic number, how do you determine the number of neutrons?
Answers
Answer:
You subtract the given atomic mass by the atomic number
Explanation:
The answer is the explanation
the volume of akshay's plasma was 3l before dr. witmer administered the iv. assume that there was complete mixing of the administered water with his plasma but no mixing with his interstitial fluid. calculate akshay's plasma osmolarity after the infusion mixed with his plasma, but before any water entered his rbc.
Answers
Akshay's plasma osmolarity after the infusion mixed with his plasma, but before any water entered his rbc is 6 osmol/L.
Osmolarity is a measure of concentration that reflects the solutions' osmoticity. Osmolarity is how osmolar concentration is expressed.
The osmolarity of Akshay's plasma after the infusion can be calculated using the following formula:
Osmolarity = 2 x [solute concentration]
where solute concentration is the concentration of the solutes in the plasma.
Assuming the IV contained isotonic saline with a concentration of 0.9%, then the solute concentration of Akshay's plasma would be 0.9/1000 * 3L = 0.0027.
Therefore, the osmolarity of Akshay's plasma after the infusion would be 2 x 0.0027 = 0.0054 osmol/L.
Akshay's plasma osmolarity after the infusion mixed with his plasma, but before any water entered his RBC = (3 L x 2 Osmol/L) = 6 osmol/L.
learn more about solute Refer:brainly.com/question/24058779
#SPJ4
I currently just need help with this right now.

Answers
Answer:
2.43 grams are needed
Explanation:
I did the test
Hope this helps :)
chinese religions are ______, meaning that there is not necessarily any conflict in combining several different religious traditions or practices.
Answers
Answer:Buddhism
Explanation:
chinese religion is a Buddhism
Explain why the other types of decay are not considered fission
Answers
Answer:
As with cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events.