In part one, complete the mechanism for this cyclization reaction by adding the missing curved arrow notation. Then in part two select the common elementary steps. 5th attempt Part 1 (2 points) See Periodic Table Be sure to include any missing lone pairs and nonzero formal charges in the mechanism. Br Add curved arrows to complete the mechanistic step. Include all lone pairs and charges.
Answers
"cyclization reaction ", "curved arrow notation", "common elementary".Part 1:To complete the mechanism for this cyclization reaction by adding the missing curved arrow notation, we need to add a curly arrow from the lone pair of electrons on the oxygen atom of the -OH group to the carbon atom of the carbonyl group.
This will result in the formation of a double bond between the carbon and oxygen atoms, as shown in the figure below:Please refer to the attached image. Part 2:The common elementary steps for this cyclization reaction are:Electrophilic Attack: The electrophile attacks the nucleophile to form a new bond.Nucleophilic Attack: The nucleophile attacks the electrophile to form a new bond.Proton Transfer: A proton is transferred from one molecule to another to form a new molecule.
Learn more about cyclization reaction here:
https://brainly.com/question/28216618
#SPJ11
Related Questions
what is it called when an enzyme changes shape
Answers
Answer: Induced Fit
Explanation: The process wherein the enzymes change their shape is called as induced fit. It is the precision aligning of enzymes essential for catalytic activity which is caused by the binding of the substrate as enzymes possess active sites. Hence, as the substrate approaches the enzymes, the enzyme alters its shape.
YOU ARE AMAZING AND YOU ARE SO IMPORTANT HAVE A GOOD DAY!
Answers
Answer:
Thx Have a fantastic day! :)
Explanation:
what is δh∘rxn for the following chemical reaction? co2(g)+2koh(s)→h2o(g)+k2co3(s)
Answers
The enthalpy change (ΔHrxn∘) of the given reaction is -1361.9 kJ/mol.
The given chemical reaction is: CO₂ (g) + 2 KOH (s) → H₂O (g) + K₂CO₃ (s)
To determine the enthalpy change of the given reaction, we need to find the difference between the products' enthalpy and the reactants' enthalpy. We use the standard enthalpy of formation, which is the energy change that occurs when one mole of a compound is formed from its elements in their standard states.
Using the following values given in the table: ΔHf∘CO₂ (g) = -393.5 kJ/mol, ΔHf∘H₂O (g) = -241.8 kJ/mol, ΔHf∘KOH (s) = -424.5 kJ/mol, and ΔHf∘K₂CO₃ (s) = -1151.2 kJ/mol.
Using the equation below:
ΔHrxn∘=∑nΔHf∘products−∑mΔHf∘reactants
We find the enthalpy change of the reaction to be:
ΔHrxn∘= -1151.2 kJ/mol - (-424.5 kJ/mol) - [(-241.8 kJ/mol) + (-393.5 kJ/mol)]
ΔHrxn∘= -1151.2 kJ/mol + 424.5 kJ/mol - 635.3 kJ/mol
ΔHrxn∘= -1361.9 kJ/mol
Learn more about enthalpy change (ΔHrxn∘) at https://brainly.com/question/14047927
#SPJ11
What happens to the energy absorbed during an endothermic reaction
Answers
Answer:
more energy is absorbed when the bonds in the reactants are broken
5.(a) Give the names and symbols of respective elements. (5)
Elements
Symbols
Elements
Pr
Platinum
Silicon
Lead
K
2
Hydrogen
Silver
Symbols
Br
Mg
Hg
Answers
Answer:
Elements:
Platinum - Pt
Silicon - Si
Lead - Pb
Hydrogen- H
Silver - Ag
Symbols:
Pr - Praseodymium
K - Potassium
Br - Bromine
Mg - Magnesium
Hg - Mercury
What are the products of the following neutralization reaction? (No need to balance)
H3PO4 + Ba(OH)2 --> ??? + ???
A.)H2O + Ba3(PO4)2
B.)H30+ + BaPO4
C.)H30+ + Ba3(PO4)2
D.)H2O + BaPO4

Answers
H3PO4 + Ba(OH)2 --> Ba3(PO4)2 + H2O
so, (A) H2O + Ba3(PO4)2 is your answer.
Which salt is the most soluble in water at 50⁰c
Answers
Answer:
Table salt, or sodium chloride (NaCl), the most common ionic compound, is soluble in water (360 g/L)
he half-life of a radioactive substance is 21 years. If we begin with a sam substance, calculate the value of b to complete the model belo which gi sample remaining after t years. f(t)=85⋅(b) t
Enter your answer for b in the box below, rounded to three decimals.
Answers
The value of b in the equation \(\( f(t) = 85 \cdot b^t \)\) represents the decay factor of the radioactive substance. To determine the value of \( b \), we can use the information that the half-life of the substance is 21 years.
The half-life of a radioactive substance is the time it takes for half of the substance to decay. In this case, the half-life is 21 years, which means that after 21 years, the amount of the substance remaining will be half of the initial amount.
We can use this information to set up an equation:
\(\(\frac{1}{2} = b^{21}\)\)
To solve for b, we need to take the 21st root of both sides of the equation:
\(\(b = \left(\frac{1}{2}\right)^{\frac{1}{21}}\)\)
Using a calculator, we can evaluate this expression:
\(\(b \approx 0.965\)\)
Therefore, the value of b in the equation \(\( f(t) = 85 \cdot b^t \)\) is approximately 0.965.
Learn more about radioactive from this link:
https://brainly.com/question/1770619
#SPJ11
Waves that move through matter only are called
waves. Waves that move through space are called
waves.
Answers
The waves that move through matter only are called mechanical waves, and the waves that move through space are called electromagnetic waves.
Mechanical waves are waves that require a medium to move. It means that these waves need a substance (solid, liquid, or gas) to travel through, for instance, ocean waves, sound waves, and seismic waves, while Electromagnetic waves (EM waves) are waves that do not need any medium to travel through. They are formed through the vibration of electric and magnetic fields. Examples of electromagnetic waves include radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
They are classified by their wavelength, frequency, and energy. EM waves are much faster than mechanical waves. They travel at the speed of light, which is roughly 299,792,458 meters per second in a vacuum. This means that they travel at approximately 186,282 miles per second. Mechanical waves, on the other hand, can only travel through mediums and at much slower speeds.
To know more about mechanical visit:-
https://brainly.com/question/1571643
#SPJ11
Which statement about copper, diamond, and silicon(IV) dioxide is correcta)Copper and silicon(IV) dioxide have similar electrical conductivityb)In diamond the carbon atoms are covalently bonded as flat sheetsc)In silicon(IV)dioxide the silicon and oxygen atoms are covalently bonded in flat sheetsd)The structure of copper includes a lattice of positive ions
Answers
Answer:
In diamond the carbon atoms are covalently bonded as flat sheets
In silicon(IV) dioxide the silicon and oxygen atoms are covalently bonded in flat sheets
The structure of copper includes a lattice of positive ions
Explanation:
Diamond and silicon(IV) dioxide are covalent network solids. Covalent network solids are made up of atoms joined to one another by covalent bonds to form a giant lattice, The solids are hard and have a very high melting point. They do not conduct electricity. So, for both diamond and SiO2, atoms are covalently bonded to yield flat sheets in a covalent bonded network solid.
Copper is a metallic substance having metallic bonds, a metallic crystal consists of positive ions and a cloud of electrons.
are there any places on earth where the mid-oceanic ridges meet the continent?
Answers
Answer:
Yes, there are places on earth where mid-oceanic ridges meet the continents. These areas are known as "continental rift zones" or "divergent plate boundaries," and they occur where tectonic plates are pulling away from each other, causing a rift or a valley to form. As the continental crust thins and stretches, it may be separated by a mid-oceanic ridge or a seafloor spreading center.
One example of a place where a mid-oceanic ridge meets a continent is the East African Rift. This is a divergent boundary where the African Plate is splitting into two parts, and a new ocean is beginning to form. The rift runs through several countries in eastern Africa, including Ethiopia, Kenya, Tanzania, and Mozambique. Another example is the Red Sea, which is a young ocean that formed as the Arabian Plate separated from the African Plate along a divergent boundary.
So, in summary, there are several places on earth where the mid-oceanic ridges meet the continents, forming rift zones or divergent plate boundaries.
3. Rank the following acids from most acidic to least acidic. Explain the ranking using the effects that lead to stabilization of the conjugate base. ogleghe он
Answers
Based on the analysis above, we can rank the acids from most acidic to least acidic is:
1. CHCl₂COOH
2. CHF₂COOH
3. CH(CH₃)₂COOH
To rank the acids from most acidic to least acidic, we need to consider the stability of their conjugate bases. A more stable conjugate base indicates a stronger acid. The stability of the conjugate base can be influenced by several factors, including the inductive effect and the resonance effect.
1. CH(CH₃)₂COOH:
The presence of the two methyl groups (–CH₃) on the α-carbon of the carboxylic acid group increases electron density through the inductive effect. This electron-donating effect destabilizes the conjugate base, making it less stable.
2. CHF₂COOH:
The presence of the electronegative fluorine atom (–F) on the α-carbon of the carboxylic acid group withdraws electron density through the inductive effect. This electron-withdrawing effect stabilizes the conjugate base, making it more stable compared to CH(CH₃)₂COOH.
3. CHCl₂COOH:
The presence of the two chlorine atoms (–Cl) on the α-carbon of the carboxylic acid group also withdraws electron density through the inductive effect. This electron-withdrawing effect is stronger than the effect of a single fluorine atom. Therefore, CHCl₂COOH has a more stable conjugate base compared to CHF₂COOH.
Learn more about acid here:
https://brainly.com/question/30086613
#SPJ4
The complete question is:
Rank the following acids from most acidic to least acidic. Explain the ranking using the effects that lead to the stabilization of the conjugate base. CH(CH₃)₂COOH, CHF₂COOH, CHCl₂COOH.
Which two elements non the periodic table are in the same period
Answers
1. In the equation CH4 + 2O2 ---> CO2 + 2H20, what is the coefficient in 2H20? How is the coefficient used?
2. In the equation CH4 + 2O2 ---> CO2 + 2H20, how is the coefficient in 2O2 used?
(Need answered Asap Please!! )
Answers
1. The coefficient in 2H2O means that there are 2 moles of that compound for every 1 mole of CH4 and CO2. If you had 1 mole of CH4 (and enough O2), you would produce 2 moles of H2O. The coefficients describe the ratio of moles in an equation.
2. It's the same for O2. There are 2 moles of O2 for every 1 mole of CH4. When reacting O2 with CH4, you need 2 moles of O2 (which is important if you need to know how much O2 to react with CH4).
3 Ca(OH)2 + 2 H3PO4 → 6 H2O + Ca3(PO4)2
How many moles of H2O would be produced if 5.2 moles of H3PO4 are reacted with excess calcium hydroxide?
2.6 moles H2O
11 moles H2O
15.6 moles H2O
7.8 moles H2O
Answers
Answer:
15.6 moles
Explanation:
For every 2 moles of H3PO4 that is reacted, 6 moles of H2O are produced.
So if 5.2 moles of H3PO4 are reacted with calcium hydroxide, calculate the moles of H2O produced by this:
5.2 moles H3PO4 * (6 moles H2O / 2 mole H3PO4) = 15.6 moles H2O
Therefore 15.6 moles of H2O would be produced if 5.2 moles of H3PO4 are reacted with calcium hydroxide.
one gallon milk is equal to how many milliliters of milk?
Answers
Answer:
3785.411784 mL
Explanation:
The table below contains the bond dissociation energies for common bonds.
Bond Dissociation energy
(kJ/mol )
C−C 350
C=C 611
C−H 410
C−O 350
C=O 799
O−O 180
O=O 498
H−O 460
Calculate the bond dissociation energy for the breaking of all the bonds in a mole of methane, CH4.
Answers
The bond dissociation energy for breaking all the bonds in a mole of methane (CH4) is 1640 kJ/mol.
To calculate the bond dissociation energy for breaking all the bonds in a mole of methane (CH4), you'll need to consider the bond dissociation energies for the C-H bond, which is provided in the table.
The methane molecule (CH4) has four C-H bonds. According to the table, the bond dissociation energy for a single C-H bond is 410 kJ/mol.
Step 1: Calculate the energy needed to break one molecule of methane by breaking all four C-H bonds:
Energy = 4 (C-H bonds) * 410 kJ/mol (bond dissociation energy for C-H)
Energy = 1640 kJ/mol
Step 2: Calculate the energy needed to break all the bonds in a mole of methane:
Energy = 1 mole of CH4 * 1640 kJ/mol
Therefore, the bond dissociation energy for breaking all the bonds in a mole of methane (CH4) is 1640 kJ/mol.
Learn more about bond dissociation energy at https://brainly.com/question/24301293
#SPJ11
1. 30 kg =___g
Idk how to do this pls help
Answers
Answer:
30000
Explanation:
all you have to do is multiply by 1000
A 1. 07 g sample of a noble gas occupies a volume of 363 ml at 35°c and 678 mmhg. Identify the noble gas in this sample. (r = 0. 08206 l×atm/k×mol)
Answers
The identity of the noble gas is the sample is Krypton
Ideal Gas lawFrom the question, we are to determine the identity of the noble gas in the sample
From the ideal gas equation, we have that
PV = nRT
∴ n = PV / RT
Where P is the pressure
V is the volume
n is the number of moles
R is the gas constant
and T is the temperature
From the given information,
P = 678 mmHg = 0.892105 atm
V = 363 mL = 0.363 L
R = 0.08206 L.atm/mol.K
T = 35 °C = 35 + 273.15 K = 308.15 K
Putting the parameters into the equation, we get
n = (0.892105 × 0.363)/ (0.08206 × 308.15)
n = 0.0128 moles
Now, we will determine the Atomic mass of the sample
Using the formula,
Atomic = Mass / Number of moles
Atomic mass of the substance = 1.07 / 0.0128
Atomic mass of the substance = 83.6 amu
The noble gas with the closest atomic mass to this value is Krypton.
Molar mass of Krypton = 83.798 amu
Hence, the identity of the noble gas is the sample is Krypton
Learn more on Ideal Gas law here: https://brainly.com/question/20212888
#SPJ12
Which statement BEST describes what happens during solvation?
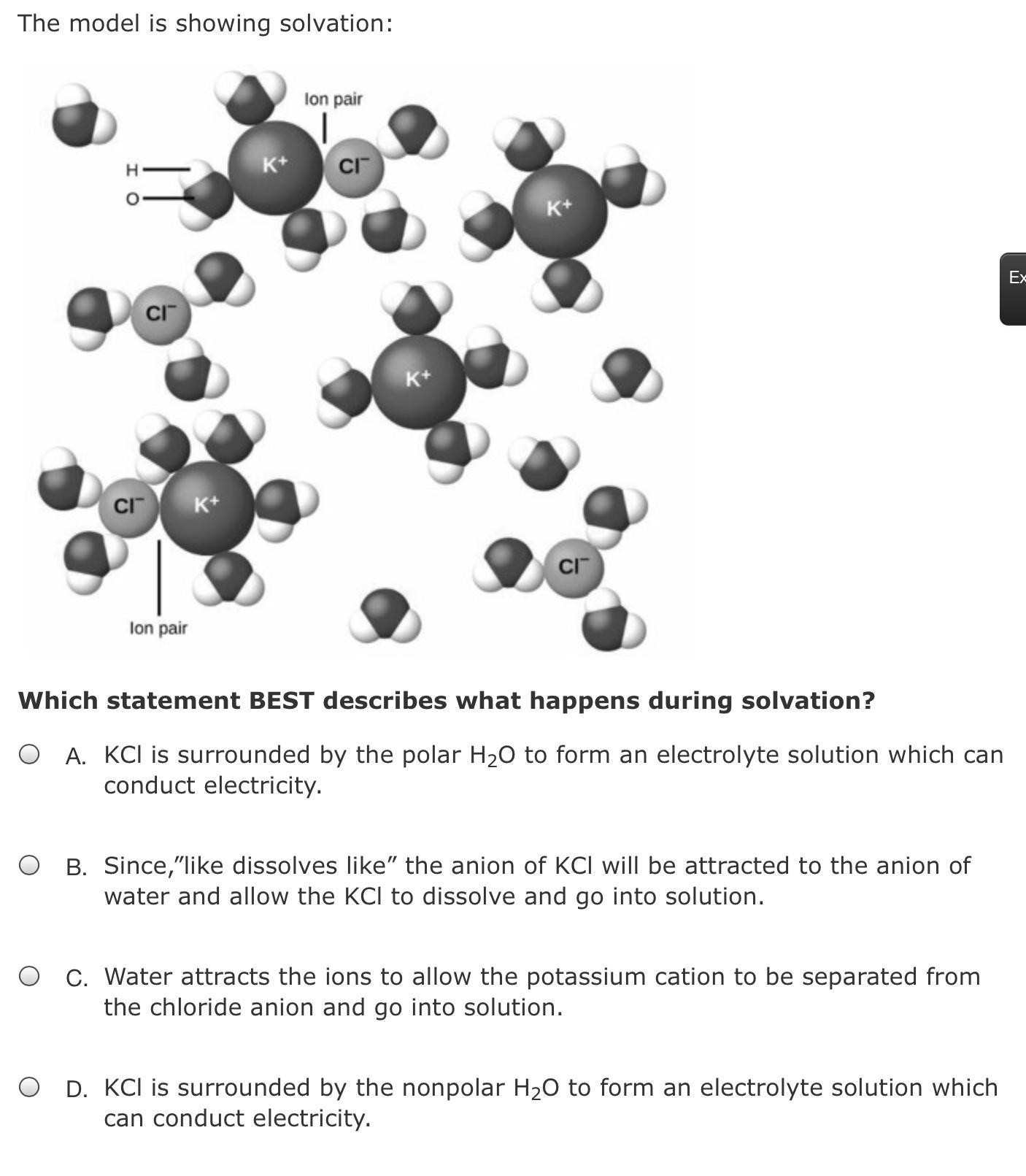
Answers
Answer:
A
Explanation:
The statement best describes solvation " KCl is surrounded by the polar water molecule to form an electrolyte solution which can conduct electricity."
What is solvation?The act of attracting and associating particles of a solvent mostly with molecules as well as ions of a solute would be known as solvation.
What is electrolyte?A material that contains ions and has been electrically conducting due to the mobility of the ions but still doesn't conduct electrons was called an electrolyte.
Whenever the solvent would be in the water, the process is referred to as hydration. The process of solvation shows the combination of solvent and solute molecules. A solute molecule is usually surrounded with solvent molecules that are organized in a special manner during solvation.
Therefore, the correct answer will be an option (A)
To know more about solvation and electrolyte
https://brainly.com/question/14356923
#SPJ3
Of the following compounds, which is the most ionic? A) SiCl4 B) BrCl C)PCl3 D) Cl2O E) CaCl
Answers
12. If a substance has a pH of 3.5, is it
acidic or basic?
Answers
pH < 7 = acidic
pH > 7 base
.
For which of the following salts would the relationship between molar solubility, s, in mol/L, and the value of Ksp be represented by the equation Ksp=4s3 ?
answer choices
PbCO3
Mg3(PO4)2
Ag2SO4
MnS
Answers
Ag2SO4 dissolves to form 2 Ag+(aq) ions and 1 SO4^2−(aq) ion. Letting s represent the molar solubility of Ag2SO4 the Ksp expression for Ag2SO4 is Ksp=[Ag+]^2[SO4^2−]=(2s)^2(s)=4s3
A substance's solubility is the greatest amount that will dissolve in a given amount of solvent at a given temperature. A specific solute-solvent combination's solubility is a defining trait, and the solubilities of various compounds can vary significantly. The saturation solution's concentration determines a solute's solubility. At a specific temperature, a saturated solution is one in which all of the solute has dissolved into the solvent. When the solute has completely dissolved in the solvent, the mixture is said to be unsaturated.
For which of the following salts would the relationship between molar solubility, s, in mol/L, and the value of Ksp be represented by the equation Ksp=4s3 ?
answer choicesPbCO3
Mg3(PO4)2
Ag2SO4
MnS
Learn more about solubility here:
https://brainly.com/question/28170449
#SPJ4
Uranus is less than times as far from the sun as Saturn is
Answers
The answer is 3 times
Edit: I'm so sorry if this is incorrect!!!
Whether a hydrogen-based energy system is environmentally cleaner than a fossil fuel system depends on ________. the car driven governmental incentives for research the amount of fossil fuels that are invested in long-term storage the source of oxygen used for the process the source of energy used to produce the hydrogen
Answers
A hydrogen energy system is made up of hydrogen production, transportation, storage, utilization etc. Whether a hydrogen-based energy system is environmentally cleaner than a fossil fuel system depends on the source of energy used to produce the hydrogen.
Hydrogen fuel cells often makes electricity by using hydrogen and oxygen atoms. The hydrogen is said to reacts with oxygen across an electrochemical cell.Hydrogen is known to carry some amount of energy carrier and can also deliver or store a large amount of energy. Hydrogen is often used in fuel cells to generate electricity, or power and heat.
Learn more from
https://brainly.com/question/3818781
Iupac name for this?

Answers
Answer:
Explanation:
4,5 diethyl-2 fluoro-3-methylheptanal
Khrushchev installed nuclear missiles in Cuba for which of the following reasons?A) deter an invasionB) The Peace CorpsC) waging the Cold WarD) quitely negotiating
Answers
Khrushchev installed nuclear missiles in Cuba primarily for reason A) to deter an invasion.
This action was taken during the Cold War, a period of heightened tension between the United States and the Soviet Union. By placing missiles in Cuba, Khrushchev aimed to establish a strategic advantage and deter potential US aggression towards both Cuba and the Soviet Union.
This move was a way of waging the Cold War (C) by showcasing Soviet military power and protecting their ally, Cuba. The Peace Corps (B) and quietly negotiating (D) are not directly related to the installation of missiles in Cuba. The presence of nuclear missiles in Cuba led to the Cuban Missile Crisis, a pivotal moment in Cold War history that brought the world to the brink of a nuclear conflict. Hence, the correct answer is Option A.
Learn more about Cold War here: https://brainly.com/question/25774915
#SPJ11
in the symbol 3p4
a. the 3 represents the principal energy level
b. the p represents the principal energy level
c. the 4 represents the principal energy level
d. all of the above
help!
Answers
Answer:
a. the 3 represents the principal energy level
Explanation:
3 is the principal energy level. The p is the sublevel. 4 is the possible occupying electron.
Which among the following pairs is inefficient as buffer pair?
A) ammonium chloride and ammonium hydroxide
B) sodium chloride and sodium hydroxide
C) boric acid and sodium borate
D) potassium carbonate and potassium bicarbonate
E) potassium fluoride and hydrofluoric acid
Answers
Among the given pairs, the pair that is inefficient as a buffer pair is (B) sodium chloride and sodium hydroxide.
A buffer solution is a solution that resists changes in pH when small amounts of acid or base are added to it. It consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. For a pair to act as an efficient buffer, it needs to have a weak acid and its conjugate base or a weak base and its conjugate acid. In the case of sodium chloride (NaCl) and sodium hydroxide (NaOH), both compounds are strong electrolytes that dissociate completely in water. Sodium chloride dissociates into Na+ and Cl-, while sodium hydroxide dissociates into Na+ and OH-. Since neither NaCl nor NaOH have acidic or basic properties, they do not possess a weak acid-conjugate base or weak base-conjugate acid pair. Therefore, they cannot act as an efficient buffer pair. In contrast, options A, C, D, and E involve pairs that consist of a weak acid and its conjugate base or a weak base and its conjugate acid, making them suitable as buffer pairs. They can effectively maintain the pH of a solution when small amounts of acid or base are added. In summary, among the given options, the pair (B) sodium chloride and sodium hydroxide is inefficient as a buffer pair because both compounds are strong electrolytes without a weak acid-conjugate base or weak base-conjugate acid relationship.
Learn more about buffer solution here:
https://brainly.com/question/16023983
#SPJ11
The solubility of water is 0.13g/L at 90kPa. What is the solubility when the pressure of the gas is increased to 150 kPa? Assume the temperature remains constant.
Answers
Answer:
Explanation:
The solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This relationship is known as Henry's Law. According to Henry's Law, the solubility of the gas in the liquid is given by:
C = k * P
where C is the concentration of the gas in the liquid (in g/L), P is the partial pressure of the gas (in kPa), and k is a constant that depends on the gas and the temperature.
To find the solubility of water at a pressure of 150 kPa, we can use the following equation:
C2 = (P2/P1) * C1
where C1 is the solubility of water at a pressure of 90 kPa (0.13 g/L), P1 is the initial pressure (90 kPa), P2 is the final pressure (150 kPa), and C2 is the solubility of water at a pressure of 150 kPa (which we want to find).
Substituting the values into the equation, we get:
C2 = (150/90) * 0.13 g/L
C2 = 0.217 g/L
Therefore, the solubility of water is 0.217 g/L at a pressure of 150 kPa, assuming the temperature remains constant.
To learn more about solubility related with temperature :
https://brainly.in/question/26349340#:~:text=Answer&text=Answer%3A,held%20together%20by%20intermolecular%20attractions.